Efficient bottom-up synthesis of new perovskite material for the production of ammonia
TOKYO,Nov. 22,2019 -- Scientists at Tokyo Institute of Technology (Tokyo Tech) found a way to synthesize a special type of perovskite that promotes the production of ammonia,which has key applications in fertilizer production and hydrogen energy. This new synthesis process can be carried out at temperatures immensely lower than that of traditional methods for the synthesis of perovskite-oxide materials and in much less time,and the produced perovskite outperforms all its competitors for producing ammonia.
Perovskites are a class of synthetic materials that have a crystalline structure similar to that of the naturally occurring mineral calcium titanate. They have been the subject of many studies because they exhibit exciting and unique properties that can be tuned according to their composition. One of their potential applications is as catalysts for the synthesis of ammonia. In other words,specific perovskites can be placed inside a reaction chamber with nitrogen and hydrogen to promote the reaction of these gases to form ammonia.
Ammonia is a useful substance that can be employed in the production of fertilizers and artificial chemicals,and even as a clean-energy carrier (in the form of hydrogen),which may be key in eco-friendly technologies. However,there are various challenges associated with the synthesis of ammonia and perovskites themselves.
The synthesis rate for ammonia is generally limited by the high energy required to dissociate nitrogen molecules. Some researchers have had some success using precious metals,such as ruthenium. Recently,perovskites with some of their oxygen atoms replaced by hydrogen and nitrogen ions have been developed as efficient catalysts for ammonia synthesis. However,the traditional synthesis of perovskites with such substitutions usually has to be carried out at high temperatures (more than 800°C) and over long periods of time (weeks).
To address these issues,in a recent study carried out at Tokyo Tech,a group of researchers led by Prof. Masaaki Kitano devised a novel method for the low-temperature synthesis of one of such oxygen-substituted perovskites with the chemical name BaCeO3−xNyHz and tested its performance as a catalyst to produce ammonia. To achieve this,they made an innovative alteration to the perovskite synthesis process. The use of Barium carbonate and Cerium dioxide as precursors (or "ingredients") involves a very high temperature would be required to have them combine into the base perovskite,or BaCeO3,because Barium carbonate is very stable. In addition,one would then have to substitute the oxygen atoms with nitrogen and hydrogen ions. On the other hand,the team found that the compound Barium amide reacts easily with Cerium dioxide under ammonia gas flow to directly form BaCeO3−xNyHz at low temperatures and in less time (Fig. 1). "This is the first demonstration of a bottom-up synthesis of such a material,referred to as perovskite-type oxynitride-hydride," explains Prof. Kitano.
The researchers first analyzed the structure of the perovskite obtained through the proposed process and then tested its catalytic properties for the low-temperature synthesis of ammonia under various conditions. Not only did the proposed material outperform most of the state-of-the-art competitors when combined with ruthenium,but it also vastly surpassed all of them when combined with cheaper metals,such as cobalt and iron (see Fig. 2). This represents tremendous advantages in terms of both performance and associated cost.
Finally,the researchers attempted to elucidate the mechanisms behind the improved synthesis rate for ammonia. Overall,the insight provided in this study serves as a protocol for the synthesis of other types of materials with nitrogen and hydrogen ion substitutions and for the intelligent design of catalysts. "Our results will pave the way in new catalyst design strategies for low-temperature ammonia synthesis," concludes Prof. Kitano. These findings will hopefully make the synthesis of useful materials cleaner and more energy efficient.
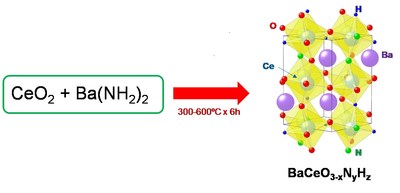
Figure 1. Synthesis of the proposed perovskite: This new protocol for the production of BaCeO3−xNyHz can be carried out at much lower temperatures and in much less time compared with conventional methods.
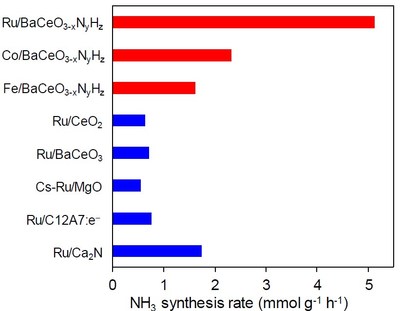
Figure 2. Synthesis rates for ammonia of various catalysts: The proposed perovskite is a much better catalyst than other state-of-the-art materials,especially when combined with more common metals,such as cobalt or iron.
Reference
Authors:
Masaaki Kitano1,2,*,Jun Kujirai1,Kiya Ogasawara1,Satoru Matsuishi1,Tomofumi Tada1,Hitoshi Abe3,4,Yasuhiro Niwa3 and Hideo Hosono1,*
Title of original paper:
Low-Temperature Synthesis of Perovskite Oxynitride-Hydrides as Ammonia Synthesis Catalysts
Journal:
Journal of the American ChemicalSociety
DOI:
10.1021/jacs.9b10726
Affiliations:
1Materials Research Center for Element Strategy,Tokyo Institute of Technology
2Precursory Research for Embryonic Science and Technology,Japan Science and Technology Agency
3 Institute of Materials Structure Science,High Energy Accelerator Research Organization
4 Department of Materials Structure Science,School of High Energy Accelerator Science,SOKENDAI
About Tokyo Institute of Technology
Tokyo Institute of Technology stands at the forefront of research and higher education as the leading university for science and technology in Japan. Tokyo Tech researchers excel in a variety of fields,such as material science,biology,computer science and physics. Founded in 1881,Tokyo Tech has grown to host 10,000 undergraduate and graduate students who become principled leaders of their fields and some of the most sought-after scientists and engineers at top companies. Embodying the Japanese philosophy of "monotsukuri," meaning technical ingenuity and innovation,the Tokyo Tech community strives to make significant contributions to society through high-impact research.
Website: https://www.titech.ac.jp/english/

View original content to download multimedia:/news-releases/efficient-bottom-up-synthesis-of-new-perovskite-material-for-the-production-of-ammonia-300962931.html