Lianhua Qingwen Receives Product Listing Approval from Singapore HSA
SHIJIAZHUANG,China,May 7,2020 -- Yiling Pharmaceutical (002603.SZ) announced that it has obtained the product listing approval issued by the Health Sciences Authority (HSA) of Singapore which grants Lianhua Qingwen Capsule the initial sale authorization in the country. The approval certifies and registers Lianhua Qingwen- a traditional Chinese medicine commonly used for the prevention and treatment of viral influenza- under the category of Chinese Proprietary Medicines (CPM).
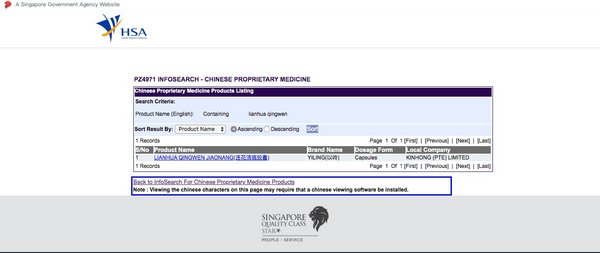
Lianhua Qingwen Receives Product Listing Approval from Singapore HAS.
Developed under the guidance of collateral disease theory,the medicine is listed as the recommended medicine for the treatment of cold and influenza by China's National Health Commission and National Administration of Traditional Chinese Medicine. Composed of 13 Chinese herbs,the TCM has been found in the clinical research that it is effective in alleviating the influenza-induced symptoms like fever,cough and fugue.
Lianhua Qingwen also plays a pivotal role in the prevention and control of COVID-19 in China. Lianhua Qingwen was shown in a clinical trial to improve the recovery rate of patients infected with COVID-19. The latest research by State Key Laboratory of Respiratory Diseases,the First Affiliated Hospital of Guangzhou Medical University has revealed that Lianhua Qingwen significantly inhibited the SARS-COV-2 replication,affected virus morphology and reduced the virus load in the infected cells in vitro.
Following the positive clinical results,the State Drug Administration of China recently approved the addition of new indications of Lianhua Qingwen in the treatment for COVID-19 symptoms. Other authorities have also incorporated the medicine as the country's standard therapy for the coronavirus pneumonia. The medicine has been listed in the "Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia" (Trial Version 4/5/6/7) issued by the General Office of the National Health and Health Commission of China and Office of the State Administration of Traditional Chinese Medicine. In addition,the Ministry of Industry and Information Technology of China has also included Lianhua Qingwen as one of the essential medical items for the COVID-19.
During the webinar organized by China's Ministry of Foreign Affair and National Health Commission with Chinese overseas students,Zhongnan Shan,China's top respiratory expert and the government's senior medical adviser,shared anti-epidemic guidance with the students and noted that Lianhua Qingwen has been proven effective for treating patients infected with COVID-19.
The efficacy of the Lianhua Qingwen has also been recognized by the global medical communities. Lianhua Qingwen is the first Chinese-patented medicine to enter FDA clinical trials for the treatment of influenza and is currently undergoing Phase II trials to evaluate efficacy and safety. To date,the Lianhua Qingwen product has been registered and granted with the sales authorization by other seven countries,including Canada,Brazil,Indonesia,Mozambique,Romania,Ecuador and Thailand.
For more information,please visit: http://en.yiling.cn/
Photo - https://photos.prnasia.com/prnh/20200507/2797588-1?lang=0