Ascentage Pharma Releases 2020 Interim Results, Reporting the Company's First New Drug Application and Advances in Global Strategic Collaborations

SUZHOU,China and ROCKVILLE,Md.,Aug. 18,2020 -- Ascentage Pharma (6855.HK),a globally focused,clinical-stage biotechnology company engaged in developing novel therapies for cancers,chronic hepatitis B (CHB),and age-related diseases,today announced its interim results for the six months ended June 30,2020. During the reporting period,Ascentage Pharma made significant strides in clinical development,external partnerships,and the building of its intellectual property portfolio,with the New Drug Application (NDA) submitted in June this year marking a key milestone for the company.
Increasing investment in innovation and further strengthening product pipeline
The company's research and development expenses for the first half of 2020 amounted to RMB240 million,as Ascentage Pharma continued to increase investment in research and development. As of June 30,2020,Ascentage Pharma has built a robust pipeline of eight clinical-stage small molecule drug candidates,with over 40 Phase I or II clinical studies being conducted in China,Australia,and the US. The company's pipeline consists of inhibitors that target key proteins in the apoptotic pathways,including Bcl-2,IAP,and MDM2-p53,to restore normal apoptotic functions; and next-generation tyrosine kinase inhibitors (TKIs) that target mutant kinases in cancers.
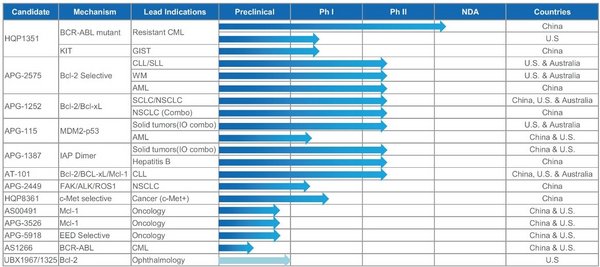
Besides the eight drug candidates in clinical development,Ascentage Pharma is further expanding its preclinical pipeline including Mcl-1 inhibitor candidate AS00491 and APG-3526,EED inhibitor APG-5918,as well as the fourth-generation BCR-ABL inhibitor AS1266 that is currently in discovery.
Advancing global clinical development and submitting the company's first New Drug Application
During the reporting period,Ascentage Pharma achieved major progress with the global clinical development of its drug candidates and submitted the company's first NDA.
Ascentage Pharma's core drug candidate,the third-generation BCR-ABL/KIT inhibitor HQP1351,has reached multiple milestones during the reporting period. In June 2020,Ascentage Pharma submitted an NDA to the Center for Drug Evaluation (CDE) of China National Medical Products Administration (NMPA) for HQP1351 in patients with T315I-mutant chronic phase chronic myeloid leukemia (CP-CML) and accelerated phase CML (AP-CML). This is the company's first ever NDA since its inception and it will likely lead to the market authorization for the first third-generation BCR-ABL inhibitor in China. A third pivotal study of HQP1351 in patients resistant/intolerant to first- or second-generation TKIs is currently ongoing and has begun patient enrollment. While advancing the global development of HQP1351 during the reporting period,HQP1351 was granted an Orphan Drug Designation and a Fast Track Designation by the US Food and Drug Administration (FDA).
The novel,orally administered Bcl-2 inhibitor APG-2575,is a key drug candidate in Ascentage Pharma's pipeline focusing on apoptosis and the first China-developed Bcl-2 inhibitor entering clinical development. Since March 2020,several Phase Ib/II studies of APG-2575 have been approved in China and the US,further accelerating the development of APG-2575 in multiple hematologic malignancies. In the US,upon receiving clearances for two clinical studies of APG-2575,Ascentage Pharma is poised to commence two global Phase Ib/II trials of APG-2575 including one of APG-2575 as a single agent or in combinations for the treatment of relapsed/refractory chronic lymphocytic leukemia or small lymphocytic lymphoma (r/r CLL/SLL); and the other for the treatment of Waldenström macroglobulinemia (WM). Of those two studies,the study in r/r CLL/SLL has dosed the first patients in the US shortly after receiving the clearance. Following the recent approval from the CDE of NMPA in China,Ascentage Pharma will soon initiate a Phase Ib study of APG-2575 as a single agent or in combination for the treatment of relapsed/refractory acute myeloid leukemia (AML),and a Phase Ib/II study of APG-2575 as a single agent or in combinations for the treatment of r/r CLL/SLL. Furthermore,the US FDA has granted APG-2575 an Orphan Drug Designation for the treatment of WM in July 2020.
Moreover,Ascentage Pharma has made significant progress with other assets in its pipeline,including APG-1252,a Bcl-2/Bcl-xL dual inhibitor; APG-115,a MDM2-p53 inhibitor; and APG-1387,an IAP antagonist. A number of datasets of those drug candidates were accepted by this year's American Society of Oncology (ASCO) and American Association of Cancer Research (AACR) annual meetings,further signifying the global recognition of the company.
Expanding global strategic collaborations and actively exploring potential for combination therapies
While building a strong internal research and development team,Ascentage Pharma has effectively developed and maintained partnerships with multinational pharmaceutical companies,biotech companies,and academic institutions. During the reporting period,the company entered multiple global collaboration agreements to explore combination therapies in a range of indications.
In June,the company entered a global clinical collaboration with Acerta Pharma,the hematology research and development center of excellence of AstraZeneca to evaluate the combination of APG-2575 with the BTK inhibitor CALQUENCE® (acalabrutinib) in patients with r/r CLL/SLL.
In July,the Ascentage Pharma entered a clinical collaboration with MSD to evaluate the combination of APG-115 and KEYTRUDA® (pembrolizumab) for the treatment of patients with advanced solid tumors.
In addition,the company signed a strategic cooperation agreement with China National Clinical Research Center for Hematologic Diseases in July on the joint construction of the "National Clinical Research Center for Hematologic Diseases Ascentage Research Institute" to promote the research and clinical development in the hematology field.
The continued expansion of the company's global collaboration network further elevates the company's global recognition and brand awareness,provides the company with access to leading drugs and candidates.
Building a global IP portfolio and solidifying leadership position in the industry
Intellectual property rights are of vital importance to Ascentage Pharma,a China-based innovative biopharmaceutical company with a global footprint. Utilizing its robust R&D capabilities,Ascentage Pharma has strategically developed a global intellectual property portfolio with exclusive licenses to issued patents or patent applications worldwide with respect to the company's product candidates. As of June 30,the company has 96 issued patents and more than 300 patent applications globally,among of which,80 patents are issued overseas.
"In midst of the COVID19 pandemic that rampaged the world in the first half of 2020,we have overcome numerous challenges and achieved breakthroughs on many fronts. The submission of the first NDA since the company's inception marked a major milestone ushering Ascentage Pharma's transition from clinical-stage to commercialization. Our continued effort to advance global clinical development of APG-2575 and other assets in the apoptosis-focused pipeline has delivered marked progress,with clinical data of these products making frequent appearances at major scientific congresses,signifying the company's growing scientific influence globally. We have advanced global development and partnerships,and have entered into collaboration agreements with multinational pharmaceutical companies and academic institutions including MSD,AstraZeneca,and China National Clinical Research Center for Hematological Diseases," said Dr. Dajun Yang,Chairman & CEO of Ascentage Pharma. "Moving forward,we will continue to enhance our research and development capabilities,strengthen our global IP portfolio,and solidify our leadership position in the industry. We aim to accelerate the global clinical development of our pipeline assets and expedite the commercialization of HQP1351,in fulfilling our mission of 'addressing unmet clinical needs in China and around the world'. In the meantime,we will remain vigilant of our financial health to safeguard the interest of our investors."
About Ascentage Pharma
Ascentage Pharma (6855.HK) is a globally,CHB,and age-related diseases. On October 28,2019,Ascentage Pharma was listed on the Main Board of the Stock Exchange of Hong Kong Limited with the stock code: 6855.HK.
Ascentage Pharma focuses on developing therapeutics that inhibit protein-protein interactions to restore apoptosis,or programmed cell death. The company has built a pipeline of eight clinical drug candidates,including novel,highly potent Bcl-2,and dual Bcl-2/Bcl-xL inhibitors,as well as candidates aimed at IAP and MDM2-p53 pathways,and next-generation tyrosine kinase inhibitors. Ascentage Pharma is also the only company in the world with active clinical programs targeting all three known classes of key apoptosis regulators. The company is conducting more than 40 Phase I/II clinical trials in the US,and China. HQP1351,the company's core drug candidate developed for the treatment of drug-resistant chronic myeloid leukemia has been granted Orphan Drug and Fast Track designations by the US Food and Drug Administration (FDA),and a New Drug Application for the drug candidate has been submitted in China. APG-2575,another one of the company's key drug candidates was recently granted an Orphan Drug Designation by the US FDA.
Forward-Looking Statements
The forward-looking statements made in this article relate only to the events or information as of the date on which the statements are made in this article. Except as required by law,we undertake no obligation to update or revise publicly any forward-looking statements,whether as a result of new information,future events,or otherwise,after the date on which the statements are made or to reflect the occurrence of unanticipated events. You should read this article completely and with the understanding that our actual future results or performance may be materially different from what we expect. In this article,statements of,or references to,our intentions or those of any of our Directors or our Company are made as of the date of this article. Any of these intentions may alter in light of future development.
Photo - https://photos.prnasia.com/prnh/20200818/2888456-1-a?lang=0
Logo - https://photos.prnasia.com/prnh/20190522/2474918-1-LOGO?lang=0