Daewoong Pharmaceutical announces Korea's first-ever SGLT2 inhibitor for diabetes treatment
Daewoong Pharmaceutical's enavogliflozin to demonstrate remarkable blood glucose lowering effect and favorable safety in type 2 diabetic patients through phase 2 clinical trial
Presented and demonstrated the result of phase 2 clinical trial in Korean patients with type 2 diabetes at the international 2020 ICDM
Aims to accelerate R&D for global market expansion through establishment of comprehensive partnerships abroad
SEOUL,South Korea,Sept. 28,2020 -- Daewoong Pharmaceutical (Daewoong) (CEO Sengho Jeon) announced the result of its phase 2 clinical trial on enavogliflozin,SGLT2 inhibitor for diabetes currently in the process of development,for the first time at the 2020 International Congress of Diabetes and Metabolism (ICDM) held on Sep. 18-19.
The 2020 ICDM,an international conference sponsored by the Korean Diabetes Association (KDA),was held for the tenth time this year. It was organized as an online event to prevent the spread of COVID-19.
When enavogliflozin was administered for 12 weeks in type-2 diabetic patients with inadequate glycemic control by diet and exercise,the patients showed statistically significant decreases in their glycated hemoglobin (HbA1c) levels in comparison to the placebo starting from week 4. In week 12,the patients' glycated hemoglobin levels decreased approximately 0.9%p in comparison to the placebo. This is a statistically significant result and indicates an additional decrease in the glycated hemoglobin by approximately 0.2–0.3%p compared to SGLT2 inhibitors of other companies for which trials were conducted in subjects in Western countries. This result,as such,raises expectations for further studies.
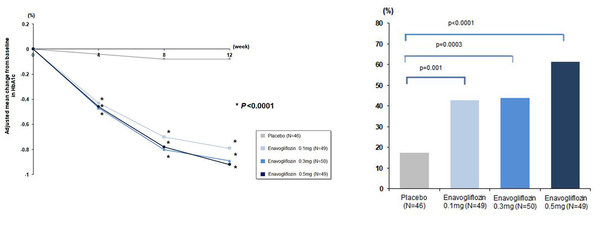
Changes in HbA1c from the baseline through 12-week administration (left) Percentage of Patients Displaying HbA1c<7.0% in Week 12 (right) *Glycemic control goal in American Diabetes Association
The percentage of patients whose glycated hemoglobin level under 7.0%* in week 12 as therapeutic response was as high as 61%,which was an increase by more than 20%p from those in the trials conducted on existing SGLT2 inhibitors. In addition,the percentage of patients achieving the HbA1c reduction at least 0.5% at week 12 was recorded as 72% at most,indicating a notable drug effect to lower serum glucose level,when considering non-responders in other SGLT2 inhibitors.
Moreover,the proportion of patients with genital infection and urinary tract infections,adverse events that can occur according to the mechanism of SGLT2 inhibitors,was only 2%,verifying outstanding safety of the drug. This is a significantly lower rate compared to the rate of 5–10% with the SGLT2 inhibitors of other companies.
Dr. Kyongsoo Park,professor of the Department of Internal Medicine at Seoul National University Hospital who served as the coordinating investigator for this trial,said,"With the result of the clinical trial to evaluate the efficacy and safety of enavogliflozin compared with placebo through monotherapy for at least 12 weeks to about 200 Korean subjects,the excellent effect to lower blood glucose level and safety of the drug were verified." "If the excellent glucose lowering effect and favorable safety of enavogliflozin are confirmed through monotherapy or combination therapy in phase 3,it is expected that enavogliflozin will be used as a good treatment option for the type-2 diabetic patients," he added.
CEO Sengho Jeon at Daewoong said,"As the excellent effect and safety of enavogliflozin were verified through the phase-2 clinical trial,we will dedicate greater efforts to developing the best-in-class SGLT2 inhibitor." "We will accelerate R&D with a goal of global market expansion by establishing comprehensive partnerships abroad," he added.
After completing the phase-2 clinical trial on a type-2 diabetes drug of enavogliflozin in Korea,Daewoong plans to initiate phase-3 clinical trials to obtain the multiple indications for type-2 diabetic treatment within this year. The company has established a goal to release the drug in Korea by 2023.
The application of SGLT2 inhibitors is currently expanding to the treatment of heart failure and chronic renal failure. Therefore,the indications for enavogliflozin are expected to expand to a wide range of diseases as well,including obesity,heart diseases,and kidney diseases in addition to diabetes. The scale of diabetic drug market in major countries around the world is predicted to grow from KRW 17 trillion in 2019 to approx. KRW 20 billion by 2024.
[Terminology]
Glycated hemoglobin (HbA1c): An indicator to check the average blood glucose level for approximately three months and is used as basic information in assessing the conditions of diabetic patients. The glycated hemoglobin level of a normal person is less than 6%,and the target level for diabetic patients is generally set to 7% or less.

View original content to download multimedia:/news-releases/daewoong-pharmaceutical-announces-koreas-first-ever-sglt2-inhibitor-for-diabetes-treatment-301138812.html