Antengene Announces Selinexor Added to Multiple Treatment Regimens in 2021 CSCO Guidelines

SHANGHAI and HONG KONG,May 6,2021 -- AntengeneCorporation Limited ("Antengene",SEHK: 6996.HK),a leading innovative biopharmaceutical company dedicated to discovering,developing and commercializing global first-in-class and/or best-in-class therapeutics in hematology and oncology,today announced that the Chinese Society of Clinical Oncology (CSCO),the most prominent organization in oncology in China,added multiple selinexor regimens to its 2021 Diagnosis and Treatment Guidelines (CSCO Guidelines) for treatment of multiple myeloma and lymphoma. Three selinexor regimens recommended by the Guideline for the Diagnosis and Treatment of myeloma include: (i) selinexor plus dexamethasone; and (ii) selinexor plus dexamethasone plus bortezomib; and (iii) selinexor plus dexamethasone plus pomalidomide for the treatment of relapsed myeloma. Meanwhile,the guideline has also recommended selinexor for the treatment of relapsed or refractorydiffuse large B-cell lymphoma (rrDLBCL). As the gold standard guiding Chinese oncologists in their clinical practice,the CSCO Guidelines is the one of the most recognized guidelines in China.
Multiple myeloma (MM) is a malignancy caused by the dysregulated proliferation of plasma cells. It is the second most common hematologic malignancy in many countries. MM is hard to treat and prone to relapse. Despite availability of a number of treatments for relapsed patients,most still succumb to their disease and new treatment options are needed. As a growing number of novel therapies enter into clinical treatment,it has become a challenge to choose the most effective treatment option.Diffuse large B-cell lymphoma (DLBCL)is an aggressive hematologic malignancy that about half of patients with DLBCL will not reach complete remission after receiving first-line treatments,and approximately 60% of patients with rrDLBCLlack effective treatment options.
Selinexor is the world's first approved oral selective inhibitor of nuclear export (SINE),representing a novel mechanism of action (MoA) in cancer therapy. Selinexor induces the apoptosis of cancer cells through the targeted inhibition of the nuclear export protein XPO1 that leads to the nuclear storage and activation of tumor-suppressor proteins and other growth-regulating (GR) proteins,and by down-regulating the levels of various oncogenic proteins.
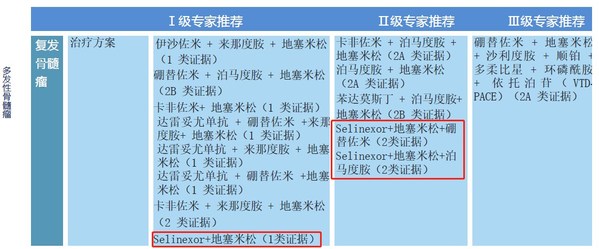
Myeloma
For the treatment of relapsed myeloma
Level 1 recommendation for selinexor plus dexamethasone (based on Class 1 evidence)
Level 2 recommendation for selinexor plus dexamethasone plus bortezomib (based on Class 2 evidence)
Level 2 recommendation for selinexor plus dexamethasone plus pomalidomide (based on Class 2 evidence)
The STORM trial is a multicenter,single-arm,open-label trial designed to evaluate the efficacy of selinexor plus dexamethasone (Xd) in patients with relapsed or refractory multiple myeloma (rrMM)that had previous exposure to multiple lines of therapy. Results from this trial showed an objective response rate (ORR) of 26% in rrMM with the Xd combination regimen in patients with rrMM who have received a median of seven previous regimens.
The STOMP trial is a multicenter,open-label,randomized Phase I/II trial designed to evaluate the safety and efficacy of various Xd combination regimens in patients with rrMM. Results from this trial showed a median progression-free survival (mPFS) of 12.2 months in patients that received the combination of selinexor,pomalidomide,and dexamethasone,and an ORR of 60% in patients that received selinexor at recommended Phase II dose (RP2D). In proteasome inhibitor (PI) nonrefractory patients,the combination of selinexor,bortezomib,and dexamethasone has demonstrated an ORR of 84%.
Lymphoma
The guideline has also recommended selinexor for the treatment of lymphoma. In the comments on the treatment of patients with rrDLBCL,the guideline recommended second-line therapies or personalized treatments that have no cross-resistance with the combination of cyclophosphamide,hydroxydaunorubicin,vincristine,and prednisolone (CHOP). In addition,the guideline also noted that multiple novel therapies including chidamide,ibrutinib,zanubrutinib,orelabrutinib,brentuximab vedotin,anti-PD-1 monoclonal antibodies,XPO inhibitor (selinexor),BCL-2 inhibitors have all demonstrated preliminary efficacy either as monotherapy or in combinations.
The SADAL trial is a registrational trial of selinexor in patients with rrDLBCL. Results of this trial demonstrated an ORR of 28.3% and a complete response (CR) of 12% in all patients,and an ORR of 34% in patients with the germinal center B-cell (GCB) subtype.
Depei Wu,MD,chairman of the Chinese Society of Hematology of the Chinese Medical Association,and director of the hematology department at the First Affiliated Hospital of Soochow University,said: "DLBCL is a highly heterogeneous hematologic malignancy,and a type of heterogeneous and invasive lymphoma with a large transformed B-cell phenotype that leads to the diffuse damage to normal lymph nodes. Patients with DLBCL commonly have high XPO1 expression that indicates a poor prognosis. The world's first approved oral XPO1 inhibitor,selinexor,has been recommend by the National Comprehensive Cancer Network (NCCN®) Guidelines for the treatment of patients with DLBCL that have received least two prior lines of therapy (including those with disease progression after transplant or CAR-T therapy). The recommendation of selinexor by the 2021 CSCO Guidelines for Diagnosis and Treatment of Lymphoma offers a new treatment option to patients. We are confident that selinexor will soon benefit more patients in need."
Wenming Chen,Chairman of the Chinese Medical Education Association Committee on Hematology,and director of the hematology department at Beijing Chao-Yang Hospital of the Capital Medical University,commented: "MM is a common type of hematologic malignancy. The survival of patients with MM has been steadily prolonged,thanks to the development and introduction of some new therapies in the recent years. But these patients still lack curative treatment and would eventually relapse after developing acquired drug-resistance. Enabled by its unique mechanism of action,selinexor can effectively address drug-resistance in MM and deliver deep response in lymphoma patients in various states of the disease. This recommendation by the CSCO Guidelines brings a new treatment option into the clinical setting. We look forward to seeing more patients benefit from selinexor."
About Selinexor (XPOVIO®)
Selinexor,a first-in-class and only-in-class oral selective inhibitor of nuclear export (SINE) compound discovered and developed by Karyopharm Therapeutics Inc. (NASDAQ: KPTI),is currently being developed by Antengene,which has the exclusive development and commercial rights in certain Asia-Pacific markets,including Greater China,South Korea,Australia,New Zealand and the ASEAN countries.
In July 2019,the US Food and Drug Administration (FDA) approved selinexor in combination with low-dose dexamethasone for the treatment of relapsed or refractory multiple myeloma (rrMM) and in June 2020 approved selinexor as a single-agent for the treatment of relapsed or refractory diffuse large B-cell lymphoma (rrDLBCL). In December 2020,selinexor also received FDA approval as a combination treatment for multiple myeloma (MM) after at least one prior therapy. In February 2021,selinexor was approved by the Israeli Ministry of Health for the treatment of patients with rrMM or rrDLBCL and in March 2021,the European Commission (EC) has granted conditional marketing authorization for selinexor (NEXPOVIO) for the treatment of MM.
Selinexor is so far the first and only oral SINE compound approved by the FDA and is the first drug approved for the treatment of both MM and DLBCL. Selinexor is also being evaluated in several other mid-and later-phase clinical trials across multiple solid tumor indications,including liposarcoma and endometrial cancer. In November 2020,at the Connective Tissue Oncology Society 2020 Annual Meeting (CTOS 2020),Antengene's partner,Karyopharm,presented positive results from the Phase III randomized,double blind,placebo controlled,cross-over SEAL trial evaluating single agent,oral selinexor versus matching placebo in patients with liposarcoma. Karyopharm also announced that the ongoing Phase III SIENDO trial of selinexor in patients with endometrial cancer passed the planned interim futility analysis and the Data and Safety Monitoring Board (DSMB) recommended the trial should proceed as planned without any modifications. Top-line SIENDO trial results are expected in the second half of 2021.
Antengene is currently conducting five mid- or late-stage clinical trials of selinexor for the treatment of MM,DLBCL,non-small cell lung cancer,and peripheral T and NK/T-cell lymphoma. Furthermore,Antengene has submitted New Drug Applications (NDAs) for selinexor in five Asia Pacific markets including mainland China,Singapore and Hong Kong,and was granted the Priority Review status by China's NMPA and an Orphan Drug Designation by the Ministry of Food and Drug Safety of South Korea (MFDS).
About Antengene
Antengene Corporation Limited ("Antengene",SEHK: 6996.HK) is a leading clinical-stage R&D driven biopharmaceutical company focused on innovative medicines for oncology and other life threatening diseases. Antengene aims to provide the most advanced anti-cancer drugs to patients in the Asia Pacific Region and around the world. Since its establishment in 2017,Antengene has built a broad and expanding pipeline of clinical and pre-clinical stage assets through partnerships as well as in-house drug discovery,and obtained 13 investigational new drug (IND) approvals and submitted 5 new drug applications (NDA) in multiple markets in Asia Pacific. Antengene's vision is to "Treat Patients Beyond Borders". Antengene is focused on and committed to addressing significant unmet medical needs by discovering,developing and commercializing first-in-class/best-in-class therapeutics.
Forward-looking statements
The forward-looking statements made in this article relate only to the events or information as of the date on which the statements are made in this article. Except as required by law,we undertake no obligation to update or revise publicly any forward-looking statements,whether as a result of new information,future events or otherwise,after the date on which the statements are made or to reflect the occurrence of unanticipated events. You should read this article completely and with the understanding that our actual future results or performance may be materially different from what we expect. In this article,statements of,or references to,our intentions or those of any of our Directors or our Company are made as of the date of this article. Any of these intentions may alter in light of future development.
*XPOVIO®is a registered trademark of Karyopharm Therapeutics Inc..