Appealing Data of CARsgen Therapeutics' CAR-T (CT041) in Advanced Gastric Cancer Presented at ESMO
SHANGHAI,Sept. 20,2021 -- On September 19,2021,CARsgen Therapeutics (stock code: 2171.HK) disclosed the latest progress of the investigator-initiated trial (IIT) of Claudin18.2 (CLDN18.2) CAR-T (CT041) for the treatment of digestive system tumors. Results of this trial have been orally presented at the European Society of Medical Oncology Congress 2021 (ESMO 2021). The presenter was Dr. Changsong Qi from Beijing Cancer Hospital.
CT041,developed by CARsgen Therapeutics,is currently the only CLDN18.2-targeted CAR-T cell therapy that has obtained IND clearance and is under clinical trials in both China and the United States.CT041 was granted an "Orphan Drug" designation by the FDA in 2020 for the treatment of gastric cancer/gastroesophageal junction (GC/GEJ) cancer and was granted the "Orphan Medicinal Product" designation by the EMA for the treatment of gastric cancer in 2021.
Appealing Data of CLDN18.2 CAR-T in Advanced Gastric Cancer Revealed at ESMO
This trial is a multicenter open-label investigator-initiated clinical trial in China for patients with CLDN18.2+ (≥+,≥10%) digestive system tumors. This clinical trial consists of a dose escalation stage and a dose expansion stage. The primary objective of this trial is to assess the safety and tolerability of CT041 and the secondary objective is to assess the efficacy and pharmacokinetics.
As of April 8,37 patients received CT041 infusion and completed at least 12 weeks of evaluation,including 28 cases of gastric/ gastroesophageal junction cancer (GC/GEJ),5 cases of pancreatic cancer (PC) and 4 cases of other types of digestive system tumors. The cell dose levels were 2.5×108,3.75×108 and 5.0×108 CAR-T cells respectively. Approximately 84% of patients had received at least 2 prior lines of therapies and the median number of metastatic organs was 3. For the 28 patients with GC/GEJ,67.9% of the subjects had peritoneal metastases. 42.9% and 35.7% of the subjects had been exposed to anti-PD-(L)1 antibody and polykinase inhibitors respectively.
Interms of safety profile,CT041 was generally well-tolerated. No treatment-related death or immune cell therapy-associated neurotoxicity syndrome (ICANS) were reported. Approximately 95% of patients experienced CRS,all being grade 1 or 2.
For the 36 patients with target tumor lesions (GC/GEJ,PC and other types of digestive system tumors),31 subjects had different degrees of shrinkage of target lesions with an ORR of 48.6% and a disease control rate (DCR) of 73.0%.
18 GC/GEJ patients who failed at least 2 prior lines of therapy (including 8 (44% of) patients ever exposed to an anti-PD-(L)1 antibody) at the dose of 2.5×108 (recommended phase 2 dose (RP2D)) CAR-T cells achieved an ORR of 61.1%,DCR of 83.3%,median PFS of 5.6m,median DOR of 6.4m,median OS of 9.5m with a median follow up of 7.6m.
For the 28 GC/GEJ patients,subgroup analysis revealed that ORR could be maintained at 50% and above in patients with different baseline characteristics.
Historical data shows that for the GC/GEJpatients who failed at least 2 prior lines of therapy,the efficacy rate of chemotherapy is about 4% to 8%,and the efficacy rate of anti-PD-1 antibody is about 11%. Therefore,compared with other treatments for GC/GEJpatients who failed at least two prior lines of therapies,CT041 has a significant improvement of ORR. Since many patients in this phase of the trial had received anti-PD-(L)1 antibody treatment,the efficacy data disclosed indicate that CT041 may become a new treatment for advanced GC/GEJpatients.
Further data of this clinical trial is planned to be disclosed in academic journals or conferences.
In 2020,there were 480,000 new cases of gastric cancer in China,accounting for 43.9% of the total incidence globally. Moreover,there is a rising trend in the incidence of gastric cancer among young people. Major treatments for advanced gastric cancer are chemotherapy and HER2-targeted therapy,but the percentage of HER2 positive patients in gastric cancer is only 7-20%. Despite several products such as PD-1 monoclonal antibodies that have been approved for advanced gastric cancer in recent years,there are still significant needs for innovative therapies.
Professor Lin Shen of Beijing Cancer Hospital commented that,"Gastric cancer is of high incidence globally and particularly in Asia. Gastric cancer incidence in China is approximately 50% of the overall global incidence. Research and treatment options for gastric cancer are still quite limited and there are strong needs for more innovative therapies to change the treatment paradigm. Data presented at ESMO showed significant efficacy and excellent tolerability of CT041 and we hope that it could benefit more cancer patients."
Dr. Zonghai Li,Co-founder,CEO,CSO,Chairman of the Board of CARsgen Therapeutics,commented that,"I would like to express the sincere gratitude to Dr. Changsong Qi for presenting the latest clinical trial data of CT041 in ESMO 2021 and to all the other investigators and researchers involved in the development of this CLDN18.2 CAR-T,which offers new hope for the gastric cancer patients. With the mission of 'making cancer curable',we will continue our endeavours in developing more innovative technology and products for cancer patients worldwide."
CARsgen Therapeutics has applied to China NMPA for the initiation of the pivotal phase II trial of CT041 in China. In US and Europe,CT041 has obtained the Orphan Drug Designation from the FDA and the EMA. The pivotal phase II clinical trial in the United States is anticipated to initiate in 2022.
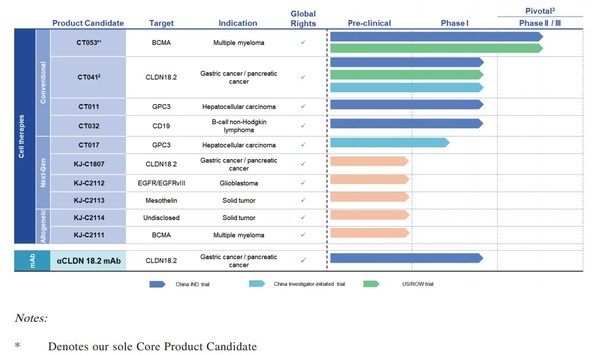
CARsgen Therapeutics currently has 11 product candidates,all of which were fully developed in house with global rights,covering conventional 2nd-generation,next-generation,and allogeneic CAR-T cell therapies,indicating a comprehensive and visionary portfolio development. CARsgen Therapeutics has obtained 7 IND approvals for CAR-T therapies in China,the United States and Canada,ranking the first among all CAR-T companies in China.
Appealing Data of CARsgen Therapeutics' CAR-T (CT041) in Advanced Gastric Cancer Presented at ESMO
(Source: https://www.carsgen.com/en/pipeline)
According to the data from Nature Biotechnology,by the end of 2019,ranked by the total number of CAR-T patents,CARsgen Therapeutics was the only Asian company among the top 20 institutes or companies globally.
In addition to existing product pipeline,CARsgen Therapeuticsstrives to continue advancing technologies centered on 4 strategic pilliars against the major challenges of the industry: 1) increasing efficacy against solid tumors 2) enhancing safety profile 3) expanding patient accessibility and 4) improving target availability. Powered by these proprietary technologies,such as CycloCAR and THANK-uCAR,CARsgen Therapeuticsplans to develop more innovative product candidates for the cancer patients worldwide.
About CT041
CT041,showed acceptable safety profile and promising antitumor activities in patients with refractory CLDN18.2 + cancer of digestive system. CARsgen Therapeutics is the first in the world to successfully identify,validate,and report CLDN18.2 and GPC3 as rational targets for CAR-T cell therapies. In addition to the investigator-initiated trials in China,we have initiated a Phase Ib/II clinical trial for advanced (unresectable or metastatic) GC/GEJ and PC in China and a Phase Ib clinical trial for advanced (unresectable or metastatic) gastric or pancreatic adenocarcinoma in the United States.
CT041 is the only CLDN18.2 targeted CAR-T cell product that has obtained IND approval globally. CT041 was granted an "Orphan Drug" designation by the FDA in 2020 for the treatment of gastric cancer or gastroesophageal junction (GC/GEJ) cancer and was granted the "Orphan Medicinal Product" designation by the EMA for the treatment of gastric cancer in 2021.
About CARsgen Therapeutics
CARsgen Therapeutics (stock code: 2171.HK) isa biopharmaceutical company with operations in China and the U.S. focused on innovative CAR-T cell therapies for the treatment of hematological malignancies and solid tumors. We have built an integrated cell therapy platform with in-house capabilities that span from target discovery,lead antibody development,clinical trials to commercial-scale manufacturing. We have internally developed novel technologies and a product pipeline with global rights to address major challenges of CAR-T cell therapies,such as improving the safety profile,enhancing the efficacy in treating solid tumors and reducing treatment costs. Our vision is to become a global biopharmaceutical leader that brings innovative and differentiated cell therapies to cancer patients worldwide and makes cancer curable.

View original content to download multimedia:https://www.prnewswire.com/news-releases/appealing-data-of-carsgen-therapeutics-car-t-ct041-in-advanced-gastric-cancer-presented-at-esmo-301380054.html