Peijia Medical's New Milestone Asia's first successful clinical case using HighLife transseptal mitral valve replacement technology
SUZHOU,China,Dec. 30,2021 -- On December 22,2021,the first clinical case using HighLife transseptal mitral valve replacement ("TSMVR") technology was successfully carried out in Asia. The implantation of Peijia's Highlife TSMVR system was performed by Professor Mao Chen and his team of West China Medical Center of Sichuan University as part of a research clinical trial. The patient is a 74-year-old female who was recently hospitalized for recurrent acute left heart failure,as well as persistent atrial fibrillation,hypertension,diabetes and other medical illnesses. The operation went smoothly with optimal positioning and good post-procedural outcome. The mitral valve regurgitation was eliminated immediately after the procedure with no LVOT obstruction. The patient has been recovering well,and was transferred from theintensive care unit(ICU) to a generalwardthe next day with normal cardiac function. She was discharged from the hospital on December 30,2021. This successful procedure laid a solid foundation for future clinical cases of Peijia's HighLife TSMVR system in China.

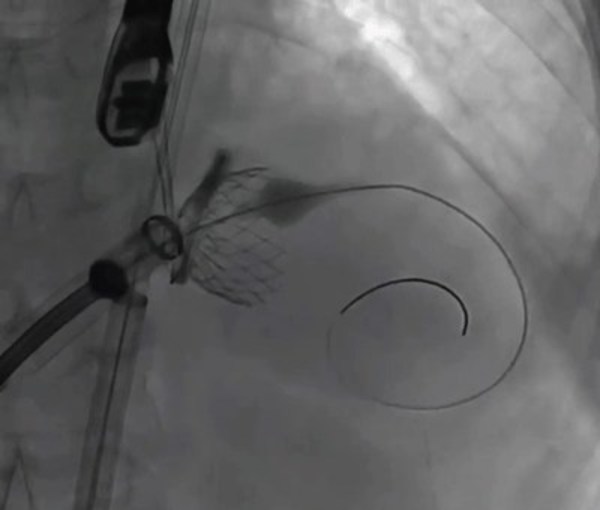
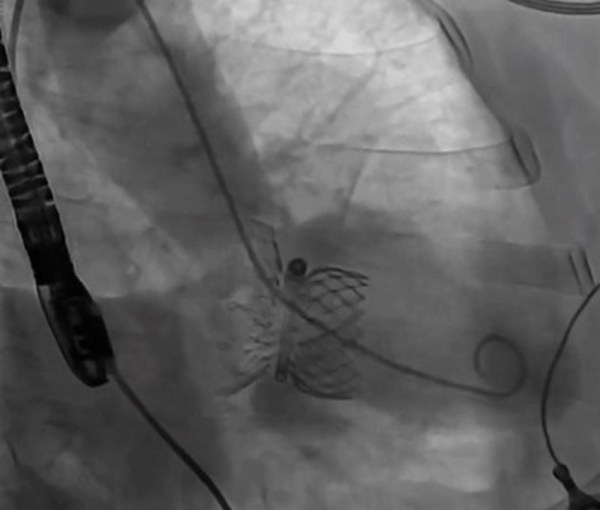
"The unique 'Valve-in-Ring' design makes it suitable for wide range of mitral valve anatomy." said Professor Mao Chen. "Most patients won't needatrial septal defect closureafter transseptal puncture with 30F delivery catheter,and the probability of vascular complications is relatively low. The procedure is performed under standard DSA in conjunction with echocardiogram,which will promote the adoption of thistechnology. I hope that this technology can be applied to more clinical uses in the near future to benefit patients with mitral regurgitation."
HighLife technology offers unique features for treating mitral valve insufficiency.
Transcatheter Mitral Valve Replacement ("TMVR") has been trending in the field of interventional therapy of structural heart disease. Early exploratory studies have proved the safety and efficacy of this technology. TMVR is suitable for wider anatomical characteristics of mitral regurgitation ("MR"). It can reduce or even completely eliminate regurgitation and the patient outcomes are usually sustainable. Furthermore,TMVR is less invasive and can be performed on elderly or high-risk patients when compared to surgical replacement.
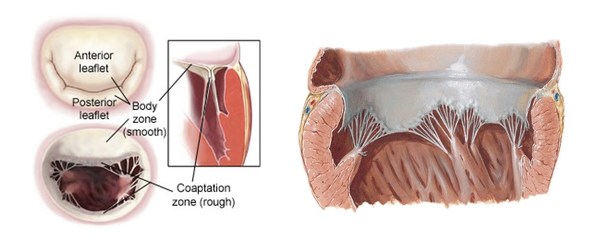
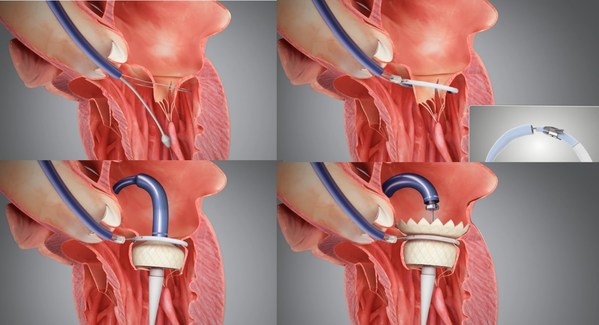
However,the field of Mitral Valve Replacement still faces many technical difficulties,including access to the target site,anchoring and the risk of paravalvular leakage ("PVL") and LVOT obstruction. Most existing approaches are either transapical or anchoring using radial force. Transapical TMVR can lead to the weakening of the left ventricular wall muscle or even a default in left ventricular beating due to surgical incision. TMVR anchoring with radial force can result in a large valve size and difficulty in delivery,which can potentially lead to left ventricularreverse remodeling. The HighLife TSMVR system employs a unique "Valve-in-Ring" concept which can better cope with these challenges. This system separates the valve from its anchoring ring and delivers the two components through the femoral vein and femoral artery respectively.
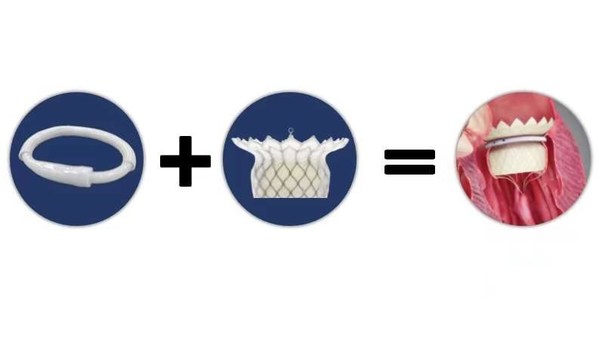
It is a simple three-step procedure. First,a guide wire loop is placed around the patient's native valve leaflets and chordae. Secondly,the anchoring ring is implanted. Finally,the self-expanding bovine pericardial valve is released through transseptal access. The delivered valve is anchored by interacting and then reaching an equilibrium position with the previously positioned ring. This allows the valve to remain in a stable position without damaging the original tissue. The procedure is relatively simple as the system is self-centering and self-aligning. The system's design helps to mitigate the risk of paravalvular leakage and effectively reduces catheter size. The procedure can be successfully completed using teleproctoring support.
Peijia Medical demonstrated its capability in international collaboration and technical expertise
In December 2020,Peijia Medical entered into a license agreement with HighLife SAS,a France based medical device company,pursuant to which HighLife SAS has granted Peijia Medical an exclusive license to develop,manufacture and commercialize certain proprietary TMVR products in the Greater China region. This technology transfer was completed in the third quarter of 2021. Local manufacturing with high quality standards in China has been established: the HighLife device produced by Peijia Medical passed all performance tests demonstrating substantially equivalent to HighLife SAS. From the start of the technology transfer to the first implantation in research clinical trial in China,Peijia Medical took less than one year to complete the process which demonstrated its capability in international collaboration and technical expertise.
To expediate the use of this world-leading technology for thebenefits of MR patients in China,Peijia Medical's consultants,Professor Nicolo Piazza and Professor Jean Buithieu from McGill University Medical Center in Canada,and the technical experts from HighLife SAS worked closely together with Peijia Medical to prepare for this clinical trial. Several training sessions involving device related and clinical practice were conducted and Cardiologists in China also actively participated in the process to ensure successful implantation.
Dr. Nicolo Piazza thought highly of this collaboration and successful implantation. "I am very glad and honored to support Professor Mao Chen and his team remotely to carry out the HighLife TSMVR procedure and share my technical experience. I was also amazed by the superb technique and tacit cooperation of Professor Mao Chen and the team. I am very happy for the successful implant of the first TSMVR system in Asia. I believed HighLife TSMVR system can benefit more patients in the future,and I look forward to more vigorous development in the field of mitral valve interventional therapy."
Adherence to its vision of "Devotion to the Heart,Reverence for Life",Peijia Medical strives to improve patients' quality of life through technological exploration and innovative persistence. "We have seen more studies on how TMVR technology targets the challenges stemming from the complex anatomy of the mitral valve and the severity of the disease. These continuous efforts signal the importance of TMVR therapy," said Dr. Michael Zhang Yi,Chairman and CEO of Peijia Medical. "Even though transseptal approach is a preferred route and excels in many ways,most existing TMVR technologies still employ a transapical approach. HighLife SAS is a global leader in the TSMVR technology,with promising clinical trial outcomes published in TCT 2021 and PCR London Valves 2021. Thanks to Professor Mao Chen and Professor Nicolo Piazza for their collaboration on the first implantation of Peijia's HighLife System,the successful implantation has further strengthened our confidence in treating mitral valve diseases with truly minimally invasive interventional technology. Peijia Medical will continue our dedication towards innovation,in the hopes that more Chinese patients who suffer from mitral valve disease can benefit from such technological advancements."
Peijia's HighLife TSMVR system represents the state-of-the-art mitral valve interventional therapy,which will greatly improve the quality of life of Chinese patients with severe MR. Peijia Medical's belief "to place patients' lives and safety at the forefront through advancing the development of minimum invasive medical therapies at home and abroad" has never changed.

View original content to download multimedia:https://www.prnewswire.com/news-releases/peijia-medicals-new-milestone-asias-first-successful-clinical-case-using-highlife-transseptal-mitral-valve-replacement-technology-301451823.html