Lunit Highlights the Effectiveness of AI in Acceleration of Immunotherapy Research--Findings to be Presented at SITC 2022

Lunit to present three abstracts featuring Lunit SCOPE IO,the company's AI biomarker for immune phenotyping.
Lunit's presentations this year will highlight the effectiveness of Lunit SCOPE IO in accelerating immunotherapy clinical trials and drug development.
SEOUL,South Korea,Nov. 7,2022 --Lunit (KRX: 328130.KQ) today announced that it will present three abstracts at the upcoming Society for Immunotherapy of Cancer's (SITC) 37th Annual Meeting,to be held in Boston,MA,and virtually from Nov. 8 – 12.
As a leading provider of state-of-the-art cancer diagnostic technology,Lunit has focused on developing novel AI biomarkers for application in immunotherapy. Since 2019,the company has released groundbreaking findings based on its AI-powered tissue analysis platform,Lunit SCOPE,demonstrating the software's predictive value in identifying patients eligible for immunotherapy.
This year,Lunit's presentations at SITC 2022 will highlight the effectiveness of Lunit SCOPE IO in accelerating immunotherapy clinical trials and drug development.
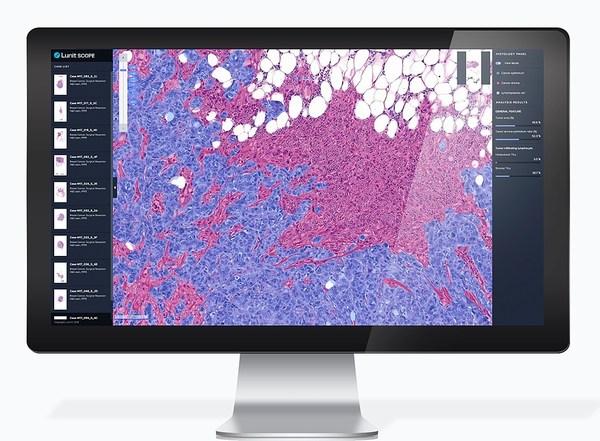
Lunit SCOPE IO,Lunit's AI biomarker for immune phenotyping
Lunit SCOPE IO analyzes a patient's cancer tissue slide image by observing the distribution of tumor-infiltrating lymphocytes (TILs) — one of the representative immunocytes that fight cancer cells. Based on the spatial distribution of TILs and cancer cells in the tumor microenvironment,Lunit SCOPE IO identifies the tissue sample as one of three immune phenotypes: inflamed,immune-excluded,or immune-desert.
One of the studies to be presented aimed to analyze the multi-cancer landscape of HER2-low expression using clinical data,pathological slides,and genetic expression registered in the National Cancer Institute's "The Cancer Genome Atlas" (TCGA).
To understand the immune microenvironment of HER2-expressing tumors,Lunit's researchers performed a spatial analysis of tumor-infiltrating lymphocytes (TILs) in association with HER2 status. As a part of this study,Lunit SCOPE IO was applied to analyze pathological slides from 7,322 patients across 22 cancer types obtained from the TCGA data set.
"The recently approved next-generation HER2 antibody-drug conjugate (ADC) has opened the door to more effective treatments for HER2-low breast cancer,which previously had limited treatment options. Based on the existing studies that HER2-expressed cancer exhibits a lower immune response,it has become important to gain more insight into the tumor microenvironment for future combinatory strategies with immune checkpoint inhibitors," explained Chan-Young Ock,Chief Medical Officer of Lunit.
"Lunit SCOPE IO,an artificial intelligence-powered H&E analyzer,can provide immune phenotypes by performing spatial analysis of tumor-infiltrating lymphocytes (TILs). This study shows Lunit SCOPE IO's possibility to further identify possible responders for the combination therapy," he added.
Lunit will also present findings from a meta-analysis study comparing the discontinuation rates of immunotherapy alone (IO-only) and immunotherapy combined with chemotherapy (chemo-IO),respectively,due to treatment-related adverse events (TRAE). 41 clinical trials,including over 13,000 patient cases,were examined. Compared to IO-only,the results indicated that chemo-IO has a significantly higher TRAE-related discontinuation rate.
TRAE-related discontinuation may lead to suboptimal treatment outcomes,warranting the discovery of a novel biomarker that can accurately predict which patients will achieve a durable response from IO-only without early treatment discontinuation. Thus,this study can be a key reference to prove Lunit SCOPE IO's clinical validity to omit the unnecessary potential risks that may result from chemo-IO.
Another study co-authored by Lunit aims to evaluate the clinical efficacy of NeoImmuneTech's NT-I7 (efineptakin alfa) and pembrolizumab to support T-cell infiltration in microsatellite-stable colorectal (MSS-colorectal) and pancreatic cancer patients with no standard treatment option.
In this clinical trial,pre-treatment and on-treatment biopsies were analyzed by Lunit SCOPE IO to objectively measure the increase in TIL infiltration. While objective measurement of TIL in small biopsied samples can be challenging,Lunit SCOPE IO is able to sensitively detect TIL in these difficult-to-analyze tissues — this indicates a practical application for the AI model in a clinical trial space.
"We are pleased to return to SITC this year to showcase new findings validating the effectiveness of Lunit's AI biomarker platform as a practical research tool for clinical study," said Lunit CEO Brandon Suh. "We will continue to work toward our goal to offer optimized treatment to cancer patients through AI."
Lunit at SITC 2022
Visit Lunit at booth #129 to get a hands-on demonstration on Lunit SCOPE IO.

View original content to download multimedia:https://www.prnewswire.com/news-releases/lunit-highlights-the-effectiveness-of-ai-in-acceleration-of-immunotherapy-researchfindings-to-be-presented-at-sitc-2022-301670202.html