Lunit to Supply AI Platform to Australia's BreastScreen NSW Machine Reading Project

Lunit's INSIGHT MMG solution has been selected for mammogram screening in Australia's BreastScreen NSW's AI evaluation and clinical integration project
Successful completion of project to be followed by 5-year operation contract with BSNSW to assist radiologists in examining 350,000 women per year
SEOUL,South Korea,Nov. 16,2022 -- Lunit (KRX: 328130.KQ),a leading medical AI provider serving over 1,000 sites around the world,today announced that it has signed a contract to supply its AI solution for the BreastScreen NSW (BSNSW) State program,managed by the Cancer Institute NSW (New South Wales' cancer control agency). Lunit's selection as the program's supplier marks the first case globally in which an AI-based solution is being deployed by a national cancer screening organization.
In 2021,the Cancer Institute NSW sought proposals and test results from potential suppliers for the supply,fulfillment,and/or delivery of a world-leading BreastScreen Machine Reading Solution built on the latest generation of deep machine learning AI technology for detecting cancers in screening mammograms. Lunit INSIGHT MMG—an AI solution for mammography analysis—was named as the preferred solution for this project.
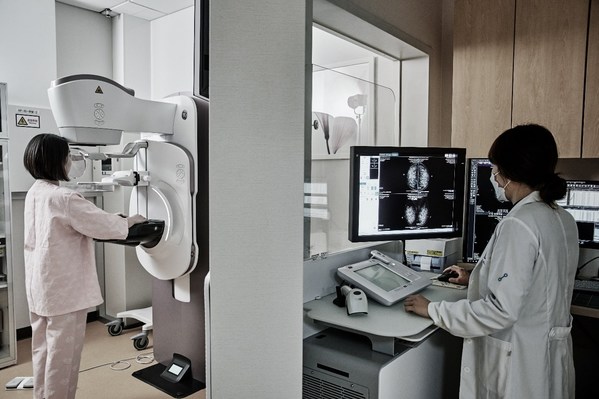
Lunit INSIGHT MMG in use in a clinical setting
BSNSW is part of the national BreastScreen Australia Program,which is jointly funded by the Commonwealth as well as state and territory governments with the aim to improve the survival rates of women with breast cancer. The service aims to detect breast cancer early,providing free mammograms to women aged 40 and over.
Phase 1 of the BSNSW Machine Reading Project will validate the Lunit INSIGHT MMG solution to confirm the potential clinical benefits prior to operational deployment. Lunit's AI solution will analyze and validate approximately 650,000 mammograms in comparison to historical reports delivered by BSNSW radiologists.
Phase 2 will conduct a prospective validation of Lunit INSIGHT MMG in the intended production workflow by assessing approximately 240,000 exams over an 8-month period. The results will be analyzed alongside radiologist reports to ensure an optimal integrated and secure workflow.
In Phase 3,operational deployment will see the Lunit INSIGHT MMG solution integrated into the BSNSW PACS environment,assisting radiologists with all screening exams for approximately 350,000 women per year. In this final phase,additional evaluations will be conducted to validate Lunit INSIGHT DBT—an AI solution for digital breast tomosynthesis—for clinical use.
Upon successful completion of all three phases,Lunit will secure a 5-year operational contract with BSNSW,which is expected to lead to further secure supply and operation throughout Australia.
"This is the first time Lunit has been awarded the opportunity to provide our AI solution for a national breast screen program," said Lunit CEO Brandon Suh. "We plan to proactively prepare for the rapidly increasing demand in national screening programs around the world,in addition to actively seeking new business opportunities."
About Lunit
With AI,Lunit aims to 'conquer cancer,' one of the leading causes of death worldwide. Lunit is an AI software company devoted to developing AI solutions for precision diagnostics and therapeutics,to find the right diagnosis at the right cost,and the right treatment for the right patients. Lunit,a portmanteau of 'learning unit,' is a deep learning-based medical AI company devoted to developing advanced medical image analytics and data-driven imaging biomarkers via cutting-edge technology.
Founded in 2013,Lunit has been acknowledged around the globe for its advanced,state-of-the-art technology and its application in medical images. Its technology has been recognized at international AI competitions surpassing top companies like Google,IBM,and Microsoft. As a medical AI company that values building clinical evidence,the company's findings are presented in major peer-reviewed journals such as the Journal of Clinical Oncology and JAMA Network Open,and global conferences including ASCO and AACR. After receiving FDA clearance and the CE Mark,Lunit INSIGHT CXR and MMG are clinically used in approximately 1,000 hospitals and medical institutions across more than 40 countries. Lunit is headquartered in Seoul,South Korea with offices and representatives in the U.S.A.,Netherlands,and China.