Promising Efficacy of AT101 CAR-T for Blood Cancer

SEOUL,South Korea,April 18,2023 --AbClon,a South Korean biotech firm,presented non-clinical and phase 1 interim results of its AT101 novel CAR-T therapy at the annual 2023 AACR conference. AT101 targets the CD19 protein for treatment of blood cancer.
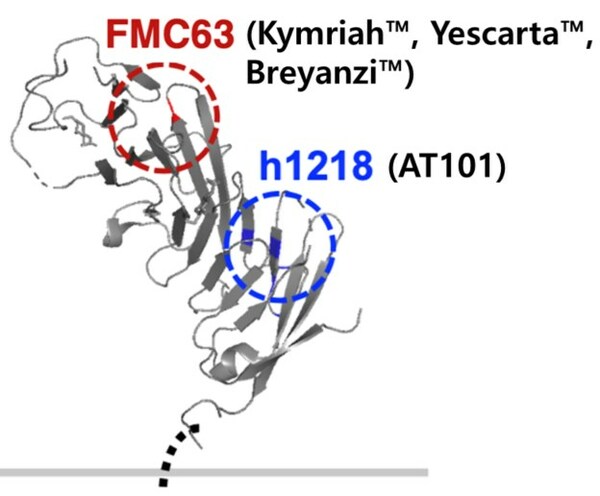
AT101 demonstrated superior anti-cancer efficacy compared to FMC63-based CAR-T therapies in non-clinical data. AT101 utilizes the h1218 antibody,developed through AbClon's Novel Epitope Screening Technology (NEST),and binds in a unique manner to CD19. AT101 demonstrated efficacy even in CD19 mutant cancer cell models that did not respond to FMC63-based CAR-T therapies. Overall,AT101 has the potential to provide new treatment opportunities for patients including those who do not respond to currently available CAR-T therapies.
An open-label,non-randomized,multicenter phase 1 study was conducted at three different dose levels in patients with relapsed / refractory B-NHL. The results of the first two dose levels in Phase 1,low and medium,have been determined. For medium dose,although the dosage administered to patients is lower than that of currently available CAR-T therapies,all three patients achieved complete response (CR) four weeks after administration. Even at 5-fold lower dosage to medium dose,CR was observed in 3 out of 6 patients and partial response (PR) was observed in 2 patients. Cytokine release syndrome (CRS) and neurotoxicity (ICANS),which appear as side effects of CAR-T therapy,were also observed at low levels of 11.1% and 22.2%. In particular,the rate of 3 or higher grade on side effects was 11.1%,showing encouraging signs with regard to safety. Clinical trial of high dosage level is currently in progress.
AT101 was administered to patients with diffuse large B cell lymphoma (DLBCL),follicular lymphoma (FL),mantle cell lymphoma (MCL),and marginal zone lymphoma (MZL). Effective responses to AT101 potentiate the application of AT101 for different types of hematologic malignancies.
Research teams led by Professor Marco Ruella of Perelman School of Medicine at University of Pennsylvania,Professor Junho Chung of Seoul National University College of Medicine,and Professor Dok hyun Yoon of Asan Medical Center are participating in this study.
AbClon owns independent intellectual property rights for the technologies applied to AT101 and is currently building channels for global expansion with local partners. AT101 patent registration has been completed in U.S.,Canada,Japan,and South Korea. Further registrations are underway in Europe and China.
Jong-Seo Lee,CEO of AbClon,said,"AT101 showed promising results in r/r B-NHL patients,including multiple CRs in the dose escalation stage and excellent safety. We are hopeful for ongoing trials of AT101 to provide more affordable and effective treatment opportunities to patients fighting blood cancer."
For more information on the ongoing trial,refer to the ClinicalTrials.gov Identifier: NCT05338931
Details of the presentation are as follows:
Presentation Title: An open label,dose escalation,phase 1 study of AT101,a novel CD19-directed CAR-T cell therapy targeting a membrane-proximal epitope of CD19,in patients with relapsed or refractory B cell non-Hodgkin lymphoma
Session Title: Phase I Clinical Trials in Progress
About AT101
AT101 is a CAR-T therapy that targets CD19 protein for blood cancer.
AbClon has initiated a Phase 1/2 clinical study of AT101 in adults with relapsed or refractory B-cell non-Hodgkin lymphoma (NCT05338931)
Phase 1/2 study is being conducted in Asan Medical Center and Ulsan University Hospital under the support of the Korea Drug Development Fund (KDDF) in Korea.
About AbClon
AbClon is a biopharmaceutical company with a robust,wholly owned R&D pipeline in oncology. It is headquartered in Seoul,South Korea. Further information about the company and its drug development programs and capabilities may be found online at https://www.abclon.com/en/