PeriCoach(r) Real-World Data Analysis Reveals Statistically and Clinically Significant Reduction in Incontinence Symptoms Within 3 Weeks

- Independent US-based biostatistician analyses PeriCoach version 3 real-world data.
- At the conclusion of the "8 Week Challenge," more than 75% of users have at least 80% reduction in leakage volume and episodes,a highly meaningful and significant improvement in continence symptoms.
- Real-world data analysis provides heightened insight into utilisation,treatment patterns and reported health outcomes for women managing urinary incontinence.
BRISBANE,Australia,Aug. 21,2018 -- Analytica (ASX:ALT).Analysis of PeriCoach real-world data conducted by an independent biostatistician reveals significant improvements in pelvic floor strength in five weeks,and reduction in urine volume and leakage episodes in only three weeks. The post-approval,all-comers observational study,reviewed women using the version 3 PeriCoach system.
PeriCoach version 3,released May 2017,comprises a state-of-the-art biofeedback system combined with a structured treatment programme. The market-leading features include real-time technique feedback to assist with proper pelvic floor muscle contraction,and force-sensors that measure the muscles that matter. During initial set-up,new users are invited to participate in a structured exercise regime and record urinary habits in the app-based bladder diary. The PeriCoach "8 Week Challenge" provides users with reminders to exercise a minimum of five sessions a week,enter information into the bladder diary three days a week,and respond to a quality of life survey at onset,four and eight weeks.
User-reported bladder diary entries wereused to analyse leakage episodes and volume. Leakage episodes weremeasured by the number of events reported per week. Leakage volume was recorded by patients using descriptors such as "light" or "full bladder," which were then mapped to an equivalent volume. More than 60% of v3 users who used the device for at least three weeks reporteda highly significant reduction in leakage episodes (30% reduction,p=.0059) and volume (30% reduction,p=.0017) by week three and beyond. By week eight,more than 75% of the users have at least 80% improvement in both episodes and volume.
"These results from PeriCoach version 3 users are absolutely clinically significant on top of being statistically significant," states Chairman Dr. Michael Monsour. "As a clinician,I hear from women daily about their frustration in lack of change in continence symptoms after years of 'Kegels at home.' This echoes data that shows performing un-assisted pelvic exercises women report outcomes of 3% almost continent,87% unchanged and 10% worse.PeriCoach provides the best of both worlds,clinical level guidance from the privacy of their own homes.Now,women can expect to have noticeable improvement in continence symptoms in just a few weeks."
Assessment of strength was conducted through measurement of direct force exerted on the vaginal sensor during each session. This is an objective measure rather than subjective digital examination common for pelvic strength assessment. PeriCoach version 3 users demonstrated week-on-week improvement in strength with nearly a third,on average,having at least a 50% improvement in strength,resulting in predictive improvement by week five (p=.004).
"Real-world data analysis is invaluable in the e-health arena. This type of data truly demonstrates how consumers actually interact with a product as well as how a product will work outside the confines of a clinical trial," said Geoff Daly,CEO of Analytica Ltd. "PeriCoach version 3 gives us the power to analyse the user experience while leveraging our understanding of key clinical outcome measures. All the enhancements and investment into the version 3 PeriCoach have been designed with this focus. The biggest success here is that women and clinicians can be confident in expectations for improvement in continence when pelvic floor rehabilitation is supported by PeriCoach."
Analytica plans to submit data for publication in urological and woman's health journals.
For more information,please contact: investorrelations@analyticamedical.com
For more information about the PeriCoach System: www.PeriCoach.com
For more information about Analytica: www.AnalyticaMedical.com
Follow us on: Facebook|Twitter|Google Plus|YouTube
About Analytica Limited
Analytica's lead product is the PeriCoach® System – an e-health treatment system for women who suffer Stress Urinary Incontinence. This affects 1 in 3 women worldwide and is mostly caused by trauma to the pelvic floor muscles as a result of pregnancy,childbirth and menopause.
PeriCoach comprises a device,web portal and smartphone app. The device evaluates activity in pelvic floor muscles. This information is transmitted to a smartphone app and can be loaded to a cloud database where physicians can monitor patient progress via web portal. This novel system enables physicians to remotely determine if a woman is performing her pelvic floor exercises and if these are improving her condition. Strengthening of the pelvic floor muscles may also improve sexual sensation or satisfaction in some women.
PeriCoach has regulatory clearance in Australia,and has CE mark and USFDA 510(k) clearance. The product is available for sale from pericoach.comin Australia,New Zealand,UK and Ireland.

PeriCoach is a kegel exerciser with a smartphone app and sensor,guiding women through three minutes workouts with proven results in weeks.
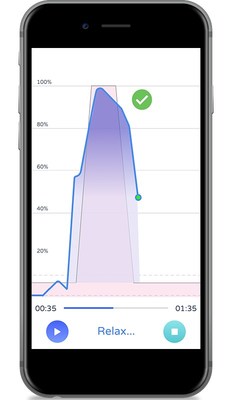
The PeriCoach technique guidance,developed in coordination with experts in pelvic health,provides visual feedback at each rep during a pelvic floor exercise session indicating proper or incorrect performance. Optimal performance is a key factor for improvements in strength and reduction in incontinence symptoms.
Video - https://www.youtube.com/watch?v=7lF-euGz9jo
Photo - http://cusmail.com/res/2023/07-23/20/bbf92594724203f8e6219cff77e1ef8a.jpg
Photo- http://cusmail.com/res/2023/07-23/20/6c4b5c33a1d191dc81f4d50cb369151a.jpg
Logo - http://cusmail.com/res/2023/07-23/20/59cab24dcc742709fe16c388967d873c.jpg