VUNO Med(R)-Fundus AI(TM) receives MFDS Regulatory Approval as Class III Medical Device
SEOUL,South Korea,April 14,2020 -- VUNO,a member company of the Born2Global Centre,announced that their AI based screening solution for the fundus,VUNO Med®-Fundus AI™ is approved as a Class III medical device by the Ministry of Food and Drug Safety. VUNO Med®-Fundus AI™ is the first ever AI device to gain a Class III approval in Korea and is clinically validated to provide highly accurate screening results.
According to the research paper published in Ophthalmology,one of the most prestigious journals in the field,the solution's clinical validation (AUROC) of the 12 findings resulted in in impressive range of 96.2 to 99.9%,and 94.7 to 98.0% with external datasets,proving its stable performance and meaningful accuracy.
VUNO recently ranked first place at the ISBI 2020 fundus images challenge,one of three most prominent international biomedical imaging competitions,gaining international recognition for its outstanding technology. VUNO demonstrated outstanding performance in 3 out of 4 task categories,firmly securing its place as the best player in the field following the winning of the same competition in 2018.
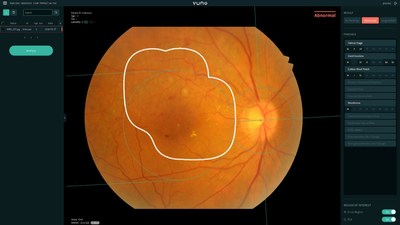
Product Screenshot of VUNO Med®-Fundus AI™
Provides all findings that are essential in diagnosing all retinal diseases,it will be a key ophthalmological solution
AI based VUNO Med®-Fundus AI™ analyzes retinal fundus images to detect and locate more than 12 lesions within one second. The 12 findings detected by the device: hemorrhage,hard exudate,cotton wool patch,drusen,membrane,macular hole,myelinated nerve fiber,chorioretinal atrophy or scar,vascular abnormality,retinal nerve fiber layer defect,glaucomatous disc change,and non-glaucomatous disc change,encompass all findings that are essential in diagnosing all retinal diseases including diabetic retinopathy,macular degeneration,and glaucoma.
The fundus examination is carried out to observe the state of the fundus,made up of the retina,optic nerve,and retinal blood vessels that play key roles in eyesight. This procedure is known to offer early detections of diabetic retinopathy,and glaucoma--three main diseases that may lead to loss of sight.
VUNO Med®-Fundus AI™ was developed based on a large-scale deep learning dataset of more than 100,000 fundus images that have been examined by more than 50 ophthalmologists including 28 specialists. The training dataset was collected from various retinal cameras (CF60Uvi,CR6-45NM,VX-10,VX-10a,nonmyd 7,and GENSIS-D).

Diagnostic Performance of VUNO Med®-Fundus AI™
Stable performance matching that of retina specialists was verified on an external validation exam incorporating multi-ethnic,multi-center settings.
For more detailed information on VUNO,visithttps://www.vuno.co/.
Media Contact
Yerim Kim
PR Manager,VUNO lnc.
rim@vuno.co
Jina Lee
PR Manager,Born2Global Centre
jlee@born2global.com

View original content to download multimedia:/news-releases/vuno-medr-fundus-aitm-receives-mfds-regulatory-approval-as-class-iii-medical-device-301039732.html