VUNO Med® LungCT AI™ Receives MFDS Regulatory Approval

SEOUL,South Korea,April 28,2020 --VUNO,a member company of theBorn2Global Centre,announced that the first CT based pulmonary nodule detecting AI solution in Korea,"VUNO Med®-LungCT AI™",was approved by theMinistry of Food and Drug Safety.The AI solution that effectively detects pulmonary nodules is set to become a valuable lung cancer diagnostic tool for healthcare professionals in Korea.
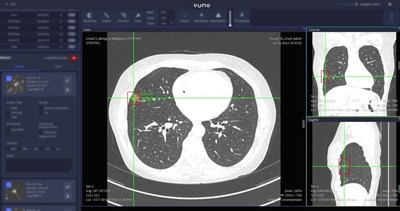
Product screenshot of VUNO Med®-LungCT AI™
This solution received PMDA (Pharmaceuticals and Medical Devices Agency)'s official approval in December 2019,proclaiming its compliance with relevant Japanese regulations,which represents a remarkable milestone for VUNO. The product is now at a full-fledged commercialization stage in Japan.
VUNO Med®-LungCT AI™ is the first medical AI solution to receive MFDS regulatory approval based on multi-center clinical trials carried out by three major hospitals,Kangbuk Samsung Hospital,the National Cancer Center,and Asan Medical Center. According to the results,this solution demonstrated high detection levels with a low false positive rate,proving its high effectiveness. It is the first Korean computer aided detection software (CADe) that detects lung nodules based on AI in chest CT,and its performance is one of the best in the world.
Proven to be the bestpulmonary lung nodule detection model,it will be effective lung cancer screening tool
Pulmonary nodules are round shadows that appear in the lungs with a diameter no larger than 3 cm,and their early detection is paramount as they may be indicative of early stage lung cancer. From August last year,the Korean government began screening for lung cancer among those in the high-risk group. There are hopes of increasing the survival rate of lung cancer patients in Korea by detecting them earlier through CT scanning. However,because more people are being scanned,combined with the high difficulty level of reading the images,many express concerns of regarding added workloads for radiologists.
VUNO Med®-LungCT AI™ detects and quantifies the size and volume of lesions based on CT imageswithin one minute. It is expected to aid the work of radiologists with improved reading accuracy and efficiency by detecting nodules that may easily be overlooked.
First,medical staff can check the preliminary information provided by the solution and with a click of the mouse,they are given further information categorized based on Lung-RADS,the official Lung CT Screening Reporting and Data System.
About VUNO Med® Solutions
A total of four VUNO AI solutions received regulatory approval by the MFDS based on clinical trial results successfully proving their effectiveness: VUNO Med®-Fundus AI™,VUNO Med®-Chest X-ray™,and VUNO Med® - BoneAge™ along with VUNO Med® Lung CT AI™. With such a robust portfolio,VUNO is on its way to establishing an unrivaled foothold across the entire process of AI medical device commercialization—product planning,technology development,regulatory approval,and clinicalvalidation—in various radiological fields including chest CT and x-ray,fundus imaging,and hand x-ray.
For more detailed information on VUNO,visithttps://www.vuno.co/.
Media Contact
Yerim Kim
PR Manager,VUNO lnc.
rim@vuno.co
Jina Lee
PR Manager,Born2Global Centre
jlee@born2global.com

View original content to download multimedia:/news-releases/vuno-med-lungct-ai-receives-mfds-regulatory-approval-301049080.html