HistoIndex's AI-based Digital Pathology Platform: A Validated Quantification Tool Recommended in the Guidelines for the Prevention and Treatment of Chronic Hepatitis B in China
SINGAPORE,May 25,2020 --Singapore medtech company HistoIndex's AI-based stain-free digital pathology platform has long been established as a suitable tool for global clinical guidelines that outline theassessment,prevention and treatment of Chronic Hepatitis B (CHB) infections. In thelatest edition of China's Guidelines for the Prevention and Treatment of Chronic Hepatitis B [1],the digital pathology platform was recommended for use in providing an automated and quantitative analysis of the liver's morphological characteristics in unstained liver biopsies with high repeatability and accuracy.
Challenges in Treating CHB Patients
Hepatitis B has been known as a complex disease to treat,due to the challenge in distinguishing patients with an active infection. These patients require intervention as opposed to patients with an inactive infection who require only standard monitoring without treatment. For patients with an active CHB infection,the treatment decision is usually based on the increased level of alanine aminotransferase (ALT) indicating an inflammation of the liver,high HBV-DNA serum level and the presence of the Hepatitis B e-antigen. However,these levels do not determine the presence and level of fibrosis in the liver.
To prevent the progression of CHB to cirrhosis or Hepatocellular Carcinoma (HCC),it is important to identify patients whose livers are affected by advanced fibrosis prior to therapy,as the post treatment prognosis depends on the stage of fibrosis. This can only be achieved with a fibrosis assessment by performing a liver biopsy,the current gold standard in evaluating the degree of inflammation,necrosis,and fibrosis in all liver conditions.
Identifying Significant Morphological Features
Besides evaluating the severity of fibrosis and inflammation in biopsies via conventional observations,it is important to accurately assess dynamic changes in the extracellular matrix of the liver,especially the various degrees of fibrous enlargement in the liver. Having a very precise measurement of these features will help clinicians to understand the stage of fibrosis in a patient and carry out a suitable treatment management plan for their patients. Enabled by Second Harmonic Generation (SHG),HistoIndex's AI-based platform has been noted in multiple publications and studies for its ability to accurately and automatically quantify changes in liver fibrosis that are both observable and subtle,and critical for treatment evaluation and modification.
In a study in 2018,fibrosis changes were evaluated with the SHG platform CHB patients before and after 78 weeks' antiviral therapy,with results encouraging the use of this tool to assess the efficacy of new anti-fibrotic therapies in clinical trials [2]. An interesting observation from this study was the reduction in fibrous septa after treatment,which showed treatment efficacy and was analyzed with precision by the SHG platform (Figure 1). Therefore,this platform will be beneficial as an assistive tool for CHB-related diagnosis,as well as for monitoring the efficacy of antiviral therapies during CHB clinical trials. This is synonymous with the SHG platform's recommendation by China's guidelines for its value in providing an automated and quantitative analysis for the assessment of CHB.
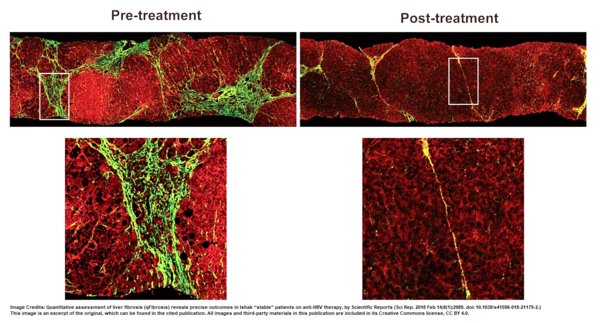
Figure 1: Stain-free images from HistoIndex’s AI-based digital pathology platform show a reduction of fibrous septa (in green) after treatment. After the images are produced,the platform generates data on the assessment of fibrosis parameters,which are crucial in determining treatment evaluation and modification.
Image Credits: Quantitative assessment of liver fibrosis (qFibrosis) reveals precise outcomes in Ishak "stable" patients on anti-HBV therapy,Scientific Reports [2]. This image is an excerpt of the original,which can be found in the cited publication. All images and third-party materials in this publication are included in its Creative Commons license,CC BY 4.0.
Says Professor You Hong*,Chief Physician and Professor at the Beijing Friendship Hospital (affiliated with the Capital Medical University) and a co-author of the 2018 study,"As a clinician,we need to make practical and reasonable decisions for our patients. Including intelligent and reliable clinical tools such as the SHG platform in the guidelines will greatly enhance our diagnostic capability and personalize our treatment options in managing patients with CHB. Having utilized this platform in our studies has helped us to observe significant changes in the vital fibrosis parameters that are crucial in determining the severity of CHB and deciding the appropriate treatment route." Professor You is a Key Opinion Leader (KOL) in the Hepatology field,and her additional appointments include Deputy Director of the Chinese Society of Hepatology and Steering Committee Member of APASL.
Dr Dean Tai,Chief Scientific Officer of HistoIndex,adds,"We have substantial data from studies conducted with the involvement of KOLs that show the potential of our AI-based platform,in the area of CHB as well as Nonalcoholic Steatohepatitis (NASH). I am confident that our platform will contribute significantly if implemented in similar international clinical guidelines,as well as in consortiums and workgroups that have been set up globally for the management of CHB."
China: Bearing a Heavy Disease Burden
As one of the countries battling CHB infections,China has the highest burden in the world,with one third of the world's infected people living in China [3]. Most of them are unaware of their infection,making this disease a truly silent epidemic. With limited access to diagnosis and treatment,these people face a bleak future as their condition will eventually progress – without treatment – to cirrhosis and eventually,liver cancer. About 10 million people living with CHB will die by 2030 [4] [5],with most of these deaths being avoidable. As part of efforts to help the nation in this devastating disease,experts under the Chinese Medical Association's (CMA) Infectious Diseases and Liver Diseases Branch released the updated guidelines in 2019.
EDITOR'S NOTES
* Professor You Hong is also the Assistant Dean at the Capital Medical University,where she holds additional appointments as Director of the Office of Academic Research and Director of the Experimental Center.
A Major and Growing Global Health Concern
According to the World Health Organization (WHO),CHB has resulted in an estimated 887,000 deaths in 2015,mostly from cirrhosis and hepatocellular carcinoma (i.e. primary liver cancer),and 257 million people are living with CHB as of the same year. As of 2016,27 million people were aware of their infection,while only 4.5 million of the people diagnosed were on treatment [6]. Following WHO's call to eliminate viral hepatitis as a public health problem,and bring down new infections by 90% and reduce deaths by 65% by 2030,countries across the world have implemented clinical guidelines to standardize and aid in the prevention,diagnosis and antiviral therapy of CHB:
(a) According to the American Association for the Study of Liver Diseases (AASLD) practice guidance,a liver biopsy should be considered in patients with persistently borderline normal or slightly elevated ALT levels,particularly those who have been infected with HBV for a long period of time and are now older than 40 years of age [7].
(b)Similarly,in the European Association for the Study of the Liver (EASL) 2017 Clinical Practice Guidelineson the management of Hepatitis B Virus Infections,patients without cirrhosis should be considered for treatment when their severity of liver disease assessed traditionally by liver biopsy shows at least moderate necroinflammation and/or at least moderate fibrosis [8].
(c) In the 2016 update of the Asian-Pacific Association for the Study of the Liver (APASL) Consensus Guidelines on Invasive and Non-invasive Assessment of Hepatic Fibrosis,the liver biopsy remains the gold standard for assessing liver fibrosis. Interestingly,the guidelines acknowledged a possible shift from routine light microscopy to digital image analysis,and the incorporation of numerical measurements in conjunction with the integrated analysis of other cellular information [9]. Since then,microscopy and analysis of liver fibrosis has indeed been transformed; pathologists today are assisted by smart digital pathology tools to make accurate and objective assessments of fibrosis and other relevant features in the liver.
References
1. Chinese Medical Association Infectious Diseases Branch,Chinese Medical Association Hepatology Branch. Guidelines for the Prevention and Treatment of Chronic Hepatitis B (2019 Edition) [J]. Chinese Journal of Clinical Infectious Diseases,2019,12 (6): 401-428.: 10.3760 / cma.j.issn.1674-2397.2019.06.001.
Note: The Chinese Journal of Clinical Infectious Diseases website is in Mandarin and is enabled with an automatic prompt that offers the option for an English translation.
2. Sun,Y.,Zhou,J.,Wu,X. et al. Quantitative assessment of liver fibrosis (qFibrosis) reveals precise outcomes in Ishak "stable" patients on anti-HBV therapy. Sci Rep 8,2989 (2018). https://doi.org/10.1038/s41598-018-21179-2
3. Shanquan Chen,Jun Li,Dan Wang,Hong Fung,Lai-yi Wong,Lu Zhao. The Hepatitis B epidemic in China should receive more attention. The Lancet Volume 391,Issue 10130,P1572,April 21,2018. https://doi.org/10.1016/S0140-6736(18)30499-9.
4. World Health Organization. Global Hepatitis Report 2017. Accessed 29 April 2020.
5. World Health Organization. Up to 10 million people in China could die from chronic hepatitis by 2030 – Urgent action needed to bring an end to the 'silent epidemic'. Accessed 29 April 2020.
6. World Health Organization,Hepatitis B Fact Sheet. Accessed 29 April 2020.
7. Terrault,N.A.,Lok,A.S.,McMahon,B.J.,Chang,K.‐M.,Hwang,J.P.,Jonas,M.M.,Brown,R.S.,Jr.,Bzowej,N.H. and Wong,J.B. (2018),Update on Prevention,Diagnosis,and Treatment of Chronic Hepatitis B: AASLD 2018 Hepatitis B Guidance.Hepatology,67: 1560-1599. doi:10.1002/hep.29800.
8. European Association for the Study of the Liver,EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. Volume 67,Issue 2,P370-398,August 01,2017. https://doi.org/10.1016/j.jhep.2017.03.021
9. Sarin,S.K.,Kumar,M.,Lau,G.K. et al. Asian-Pacific Clinical Practice Guidelines on the Management of Hepatitis B: A 2015 Update. Hepatol Int 10,1–98 (2016). https://doi.org/10.1007/s12072-015-9675-4
Media Contacts: Ms Cynthia Anne Victor,Corporate Communications & PR,HistoIndex,cynthia.victor@histoindex.com
Photo - /20200522/2811543-1?lang=0