How to Recognize Side-Effects from Immunotherapy? New NCCN Guidelines for Patients can Help

PLYMOUTH MEETING,Pennsylvania,June 29,2020 -- The National Comprehensive Cancer Network® (NCCN®) today announced the publication of new NCCN Guidelines for Patients®:Immunotherapy Side Effects--Immune Checkpoint Inhibitors. Over the past decade,immunotherapy--a treatment that helps the patient's own immune system recognize and destroy cancer cells--has become an important option for some cancers,and adds to traditional approaches like chemotherapy,radiation,and surgery. Often,immunotherapy is fairly well tolerated. However,emerging research shows immunotherapy can result in different side effects than chemotherapy,including severe adverse events. In fact,researchers have found 43% of patients have stopped treatment with immune checkpoint inhibitors (or ICIs,a common type of immunotherapy),as a result of serious side effects.1
NCCN Guidelines for Patients: Immunotherapy Side Effects
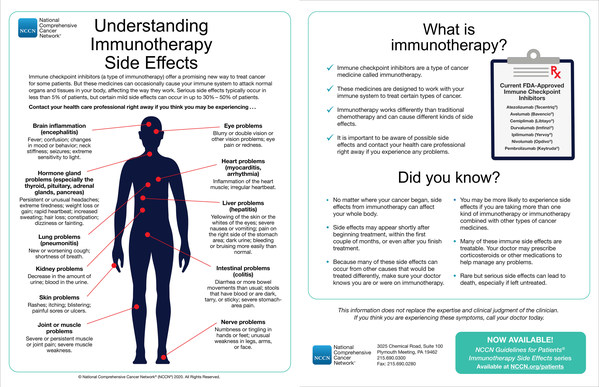
"These NCCN Guidelines for Patients are designed to educate patients and to help them recognize immune side effects so that effective interventions can be started promptly," explained Seattle Cancer Care Alliance's John A. Thompson,MD,Professor of Medicine,University of Washington,Member,Fred Hutchinson Cancer Research Center,and Chair,NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Panel for Management of Immunotherapy-Related Toxicities. "Immune checkpoint inhibitors have revolutionized our approach to the treatment of cancer. ICIs are now approved by the FDA for treating more than a dozen forms of cancer and the list is growing every year. However,by virtue of stimulating the patient's immune white blood cells,ICI therapy sometimes causes serious side-effects that mimic autoimmune disease,including significant rash and/or inflammation of the thyroid,liver,lungs,nervous system,glandular system,heart,or other organs."
The NCCN Guidelines for Patients are easy-to-understand,lay-language versions of the evidence-based,expert-consensus clinical practice guidelines used by health care providers all over the globe. They enable patients and caregivers to get a better idea of their treatment options in order to make informed decisions about their care. Features include patient-friendly illustrations,suggested questions to ask,and a glossary of terms and acronyms. A recent independent study of the NCCN Guidelines for Patients: Prostate Cancer found them to be among the most reliable and trustworthy sources for online health information.
NCCN Guidelines for Patients are available for free online at NCCN.org/patients,or via the NCCN Patient Guides for Cancer App,thanks to funding from the NCCN Foundation®. Printed copies are available at Amazon.com for a nominal fee. The immunotherapy patient guidelines have been endorsed by Be The Match/National Marrow Donor Program® (NMDP),Good Days,The Leukemia & Lymphoma Society (LLS),and Stupid Cancer.
"The Leukemia & Lymphoma Society is endorsing these NCCN materials about immunotherapy because we believe that patients should understand all aspects of their care," said Meredith Barnhart,Director of the Information Resource Center of The Leukemia & Lymphoma Society. "As patients understand more about managing toxicity,they can take better care of themselves and be prepared to ask their healthcare team the right questions to increase their quality of life."
The book stresses that immune-related adverse events can start at any point during or after immunotherapy. Most side effects can be managed effectively if identified and treated early,generally via corticosteroids. The most-common adverse effects are skin disorders. Visit nccn.org/immunotherapyguide for a quick glance at the types of adverse events that may require medical attention.
NCCN will soon add a second patient guideline on another type of immunotherapy: chimeric antigen receptor (CAR) T-cell therapy. NCCN Guidelines for Patients cover all major cancers,including breast,prostate,colon,lung,pancreatic,and many more. The immunotherapy books join other supportive care manuals for cancer-related distress and nausea.
Visit NCCN.org/patients to learn more or make a donation to the NCCN Foundation in support of these and other comprehensive,frequently-updated resources for people with cancer and their loved ones.
About the National Comprehensive Cancer Network
The National Comprehensive Cancer Network® (NCCN®) is a not-for-profit alliance of leading cancer centers devoted to patient care,research,and education. NCCN is dedicated to improving and facilitating quality,effective,efficient,and accessible cancer care so patients can live better lives. The NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) provide transparent,evidence-based,expert consensus recommendations for cancer treatment,prevention,and supportive services; they are the recognized standard for clinical direction and policy in cancer management and the most thorough and frequently-updated clinical practice guidelines available in any area of medicine. The NCCN Guidelines for Patients® provide expert cancer treatment information to inform and empower patients and caregivers,through support from the NCCN Foundation®. NCCN also advances continuing education,global initiatives,policy,and research collaboration and publication in oncology. Visit NCCN.org for more information and follow NCCN on Facebook @NCCNorg,Instagram @NCCNorgand Twitter @NCCN.
About the NCCN Foundation
The NCCN Foundation® was founded by the National Comprehensive Cancer Network® (NCCN®) to empower people with cancer and advance oncology innovation. The NCCN Foundation empowers people with cancer and their caregivers by delivering unbiased expert guidance from the world's leading cancer experts through the library of NCCN Guidelines for Patients® and other patient education resources. The NCCN Foundation is also committed to advancing cancer treatment by funding the nation's promising young investigators at the forefront of cancer research. For more information about the NCCN Foundation,visit NCCN.org/patients.
1Schadendorf D,Wolchok JD,Hodi FS,et al. Efficacy and Safety Outcomes in Patients with Advanced Melanoma Who Discontinued Treatment with Nivolumab and Ipilimumab Because of Adverse Events: A Pooled Analysis of Randomized Phase II and III Trials. J Clin Oncol 2017;35:3807-3814. Available at https://www.ncbi.nlm.nih.gov/pubmed/28841387.
Media Contact:
Rachel Darwin
267-622-6624
darwin@nccn.org
Photo - http://cusmail.com/res/2023/07-22/19/75c128ba0f0374bab9c80e7a5c643b45.jpg
Logo -https://mma.prnasia.com/media2/441768/NCCN_Logo.jpg?p=medium600