Navigating China's Medical Technology, Regulations, Quality, and Future; Hundreds of Experts Gathered at On-site Conferences of Medtec China
SHANGHAI,March 29,2021 -- Medtec China 2021 will be held in Halls 2&4 of the Shanghai World Expo Exhibition Hall on September 1–3,and the "Innovation Technology Forum and Regulation Summit" will be held at the same time.
This summit caters to the development trend and focus of China's medical device industry,and launches "Advanced Medical Device Life Cycle Risk Management" and the "Advanced Active Medical Device Technical Forum," and will also hold for the first time forums entitled "High Polymer Material Application in Medical Devices," "Precision Machining Equipment and Technology Forum," and "Core Component and Technology Seminar of Dental Products," so as to help corporate practitioners seize opportunities under the environment of continuous change and development.

Scene Picture of Medtec China 2020
Eminent Industry-University-Research Guests Gathered at Medtec China
The Concurrent Summit of Medtec China 2020 featured nearly 80 authoritative speakers and over 1,700 attendees. Speakers from government departments,industry associations,research institutes,and industry-leading enterprises,among others,provided new ideas for enterprise heads and staff from registration and regulations,through quality and compliance,to medicine,R&D and design. The content of the concurrent conference in 2021 will be even more abundant. Click to view post conference report > >

MDiT Forum and Regulation Summit 2020
Regulations,quality,and technology go hand in hand. New forum escorts product innovation
The concurrent conference on "Chinese Regulatory Updates and Compliance" will focus on the key development direction of Chinese regulations,and deliver a comprehensive and in-depth interpretation from the perspectives of industry,supervision,and enterprises to point out the direction for domestic and foreign enterprises. Meanwhile,Quality Track A will focus on "Advanced Medical Device Life Cycle Risk Management," and Quality Track B will focus on "How to respond to QMS supervision worldwide in the post-pandemic era."
Technology track will convene as many as 14 meetings,covering the medical device industry's cutting-edge technologies in the broader scope. "Advanced Active Medical Device Technical Forum," "High polymer Material Application in Medical Devices," "Overseas Advanced Medical Manufacturing Technology," and "Core Component and Technology Seminar of Dental Products" will be held for the first time. Further,"3D Materials and Technology Application in Medical Device," "Medical Device Design Forum" and other traditional dynamic meetings will once again return to bring more technical enlightenment to the participants.
Speakers are being recruited. Click to contact usto join Medtec China 2021.
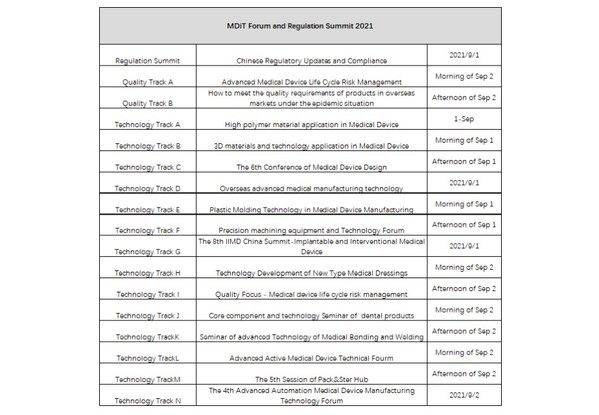
MDiT Forum and Regulation Summit 2021
*The specific schedule is subject to the site
Meetings and exhibits jointly promote the development of high-end medical equipment
As soon as the concept of high-end medical equipment and its design and manufacturing services was introduced,the Medtec China attracted many leading enterprises to join and exhibited various products suitable for high-end medical equipment.Guangzhou JU Plastic Fitting Technology Co.,Ltd.,Toradex (China) Ltd.,Matsuya Masanyi Corporation,and Noldus Information Technology have decided to participate in the exhibition and exhibit the NXP i.MX 8 computer module of precision plastic joints-Apalis iMX8,ultra-precision micro-parts,tiny needles for ophthalmology,and human engineering design,among other attractions. Click here to bookthe ideal booth > >

Part of the exhibits and exhibitors of Medtec China 2021
Medtec China 2021 will be held in Halls 2&4 of SWEECC Hall on September 1–3,gathering more than 40,000 visitors,and 500 exhibitors. The visitor pre-registration channels will be opened simultaneously on the official website and WeChat in the middle of April. For more information,please visit www.medtecchina.com