Brattea, a Leading Renal Denervation Company in China, Completes over $US20 Million Series B+ Financing, led by Kuanping Capital
SHANGHAI,April 6,2021 -- Brattea,a leading renal denervation (RDN) medical technology company in China,announced today its successful completion of over $US20 million Series B+ financing led by healthcare private equity fund Kuanping Capital. New investors include Hengxu Capital,Pudong Science & Technology Co. and Brosmed. Prior round investors include Northern Light Venture Capital,BioTrack Capital,and Sherpa Healthcare Partners.
The current Series B+ financing is the largest investment event in the RDN sector in China,which demonstrates strong confidence from the industrial and investment community. Brattea plans to accelerate clinical trial progress to maintain its leading position in RDN products and quickly expand into multiple promising indications including cancer pain and diabetes.
According to the latest statistics,the prevalence of hypertension among adults in China is over 23%,which represents nearly 300 million patients. Among them,about 50 million patients with refractory hypertension and chronic kidney disease cannot be fully treated by drugs.
Renal denervation(RDN) technology has been verified by numerous clinical trials for its efficacy and safety in treating hypertension patients. In March 2020,SPYRAL HTN-OFF MED(SPYRAL Pivotal) trial results illustrated that the blood pressure of patients in RDN surgery group was reduced by 9.2mmHg (2.5mmHg in the sham-controlled group) in the 3 months after surgery. The data showed that,without relying on antihypertensive drugs,RDN can significantly reduce blood pressure and the antihypertensive effect lasts for 24 hours,especially during the period of high incidence of cardiovascular and cerebrovascular events from midnight to early morning. Similarly,the interim results of the SPYRAL HTN-ON MED showed that for patients taking standard antihypertensive drugs,RDN still demonstrated positive results.
The real-world Global SYMPLICITY Registry indicates that systolic blood pressure could drop by 16.5mmHg based on 3-year follow-up data from over 1,700 hypertensive patients. Meanwhile,the study proved the long-term safety of RDN surgery without severe adverse events.
With the release of high-quality clinical trial results,industry leaders and regulatory agencies have begun to actively promote the product launch. Medtronic,a leading global medical device company,raised its RDN product revenue forecast at the October 2020 investor conference. Medtronic estimated that global RDN market will exceed $US1 billion by 2026 and $US3 billion by 2030. Medtronic's RDN product is expected to access the market in 2022,as the first FDA-approved product. Till the end of 2020,FDA has granted Breakthrough Device Designation to two RDN products,from Medtronic and ReCor Medical respectively.
Brattea has developed the world's first basket-shaped 6-electrode ablation catheter. The innovative design achieves excellent performance in vessel adhesion and energy release,which also avoids blood flow blocking. The ablation instrument has an intelligent operating system for temperature measurement,impedance testing and power control. The minimally invasive surgery is easy to learn and the whole procedure takes less than 40 minutes,while patient can stay awake avoiding major surgical trauma. Brattea has obtained the CE mark for RDN products and is actively seeking distribution partners in Europe.
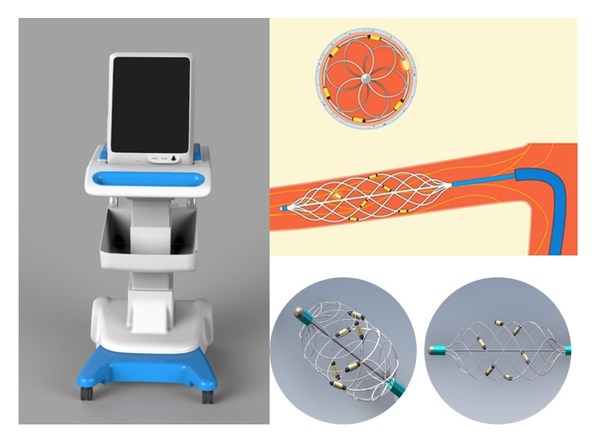
RDN is one of the few sectors that Chinese medtech companies can keep up with global leaders. Brattea RDN products have the potential to show clinical advantages compared to the Medtronic SPYRAL series and to become best-in-class globally.
The company is now enrolling a prospective,multi-center randomized controlled clinical trial to evaluate the safety and efficacy of its RDN products. The clinical trial was led by Dr. Yujie Zhou,Deputy Dean of Beijing Anzhen Hospital. Fourteen leading hospitals joined the trial,including Chinese PLA Hospital,Ruijin Hospital,West China Hospital and the First Affiliated Hospital of Zhengzhou University. The RDN product was granted into the Green Channel for innovative medical devices in 2019.
Brattea has built one of the few neuro-radiofrequency ablation platforms in the world. In addition to hypertension,its core technology has been widely applied in chronic disease management,including cancer pain and diabetes. The company created the world's first percutaneous intravascular radiofrequency nerve ablation to treat upper abdominal cancer pain. Early clinical results have shown efficacy and safety. The product has entered the clinical trial stage.
Brattea has an experienced senior management team for R&D and clinical trials. Mr. Hongguang Cao,the Founder,Chairman and CEO,is a successful entrepreneur in the medtech industry. Mr. Cao founded iRay Technology (Shanghai Stock Exchange STAR Market: 688301),Beijing WeMed and many other leading companies.
Mr. Hongguang Cao said: "Since Medtronic acquired Ardian,many globally renowned medtech companies have invested into the RDN sector. Up to 2020,clinical trials began to show good results. Brattea is dedicated to innovating in the field of energy-based intervention devices and will keep pace with global leaders."
Ms. Huihui Li,the Founding Partner and CEO of Kuanping Capital said,"RDN will be the next blockbuster product due to innovative ideas,reliable products and proven clinical results. Mr. Cao,as a successful serial entrepreneur,with his sharp product perception,rich experience,and passion for innovation will make him one of the unquestionable industry leaders. It is a great honor for Kuanping Capital to participate in this round of financing. Kuanping Capital will partner with this outstanding team to achieve great breakthroughs.
About Brattea
Founded in 2013,Brattea is a medical device research and development company rooted in China and with a global vision. The company focuses on the development and commercialization of renal denervation products with indications in hypertension,cancer pain and diabetes. Brattea has built a global IP patent portfolio and a capable R&D team in Shanghai.
About Kuanping Capital
Founded in 2016,Kuanping Capital is a healthcare dedicated private equity fund. The Kuanping team focuses on research-based,strategy-oriented active investments,and partners with outstanding entrepreneurs to achieve superior return through value creation.

View original content to download multimedia:/news-releases/brattea-a-leading-renal-denervation-company-in-china-completes-over-us20-million-series-b-financing-led-by-kuanping-capital-301261744.html