SINOMED announces the first commercial implantation and European launch of the HT Supreme Drug Eluting Stent

TIANJIN,China,Aug. 17,2021 -- SINOMED,a leading international medical device company,announced the first commercial implantation of the HT Supreme® Drug-Eluting Stent (DES) at University Hospital Galway in partnership with the National University of Ireland Galway (NUI Galway),marking the start of the European launch. The procedure was successfully performed by Professor Faisal Sharif,Professor of Translational Cardiovascular Medicine and Innovation at NUI Galway and Consultant Interventional Cardiologist at Galway University Hospitals.
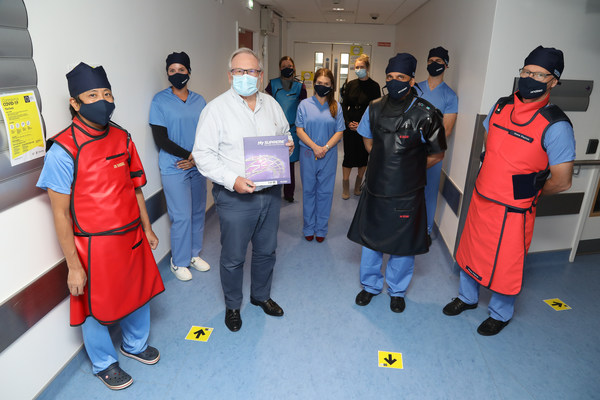
First commercial implantation of the HT Supreme DES at National University of Ireland Galway with members of the Galway University Hospital cath lab team,SINOMED and Synapse team (from left to right): Yoshinobu Onuma,Julie Aimonetti,Prof. Patrick W. Serruys,Eithne Dillion,Alicia Murray,Anna Townsend,Prof. Faisal Sharif,Robbie Dolan,Alain Aimonetti.
Professor Sharif said "We are delighted to implant the HT Supreme stent from SINOMED at University Hospital Galway in partnership with National University of Ireland Galway. Early indications show the device deliverability to be good and the novel stent technology offers faster stent endothelialisation,which will potentially reduce the need for aggressive anti-platelet treatment. Reducing the need for multiple anti-platelet treatment is an important issue especially with an aging population and multiple co-morbidities of patients who require coronary stenting."
The healing-targeted HT Supreme DES was approved for the treatment of patients with narrowing or blockages to their coronary arteries. This new device gives clinicians an additional choice of a device that is tailored to help patients accelerate their wound-healing process and restore their naturally protective vessel function after a stenting procedure.
"The HT Supreme represents a different DES technology by offering a solution specifically designed to encourage rapid healing following implantation," said Alain Aimonetti,Chief Commercial Officer,Sales,Marketing and Clinical Affairs. "This product launch is a significant landmark for SINOMED Europe as we begin the commercial phase of our operations. Additionally,we will continue our clinical commitment with further investments in the HT Supreme's robust clinical program."
"We are honored to be the first Chinese company to partner with the CORRIB Research Centrefor Advanced Imaging and Core Laboratoryfor the PIONEER IV study," said Dr. Jianhua Sun,PhD.,Chairman and Chief Executive Officer of SINOMED. "Based on previous clinical experience,the HT Supreme has been documented to be safe and effective. This new study shortens the dual antiplatelet medication for all patients,which will reduce the risk of bleeding,and assess a new method to further improve the quality of treating patients."
Prof. Sharif is one of four global Principal Investigators of the PIONEER IV trial,which is a prospective,randomised trial that will take place in 30 hospitals across Europe,enrolling 2,540 patients suffering from any type of coronary artery disease,including acute heart attack,chronic complaints or vessel narrowing. Patients eligible will undergo a non-invasive physiological vessel selection process to determine which vessel requires stenting. All patients enrolled into the trial will use the HT Supreme DES and be required to take one month dual-antiplatelet therapy after stenting.
The PIONEER IV is an investigator driven trial sponsored by NUI Galway and is centrally coordinated by the University's CORRIB Research Centre for Advanced Imaging and Core Laboratory,led by Professor Patrick W. Serruys,Established Professor of Interventional Medicine and Innovation,and Professor William Wijns,Science Foundation Ireland Professor of Interventional Cardiology.
More information on the PIONEER IV study is available at www.clinicaltrials.gov,identifier: NCT04923191.
About the HT Supreme Drug-Eluting Stent
The HT Supreme represents a novel class of stents that highlights the importance of early,timely healing. Through patented designs and proprietary processes,the HT Supreme is tailored to help patients accelerate their wound-healing process and restore their naturally protective vessel function.
About SINOMED
SINOMED is a global medical device company engaged in research,development,production,and commercial distribution of interventional devices. We are focused on developing breakthrough technologies to target unmet clinical needs in the interventional treatment of coronary,neurovascular and structural heart disease. Our mission is to expose more patients to the benefits of our medical innovations,increasing patient longevity and quality of life. For more information visit www.sinomed.com.
About National University of Ireland Galway (NUI Galway)
Established in 1845,NUI Galway is a bilingual university comprised of four colleges,19 schools,five research institutes,19,070 students,3,308 international students,2,200 staff,research collaborations with 3,267 international institutions in 114 countries,110,000 alumni,while 98% of graduates are in employment or further study within six months.
For more information visitwww.nuigalway.ie
Contact:
SINOMED B.V
Cindy Zheng
Wilhelminakade 173
3072AP Rotterdam
The Netherlands
T: +31-10-307-6295
E: cindy.zheng@sinomed-eu.com