Phillips-Medisize Increases Global Manufacturing Capacity, Capabilities and Collaborations to Drive Drug Delivery, Diagnostic and MedTech Innovations
- Medical and pharmaceutical solutions leader poised to scale manufacturing from new and expanded production facilities and R&D centers in Poland,China and U.S.
- End-to-end capabilities optimize design,development and manufacturing of world-class healthcare products and solutions while reducing time,cost and risk
-Industry-leading collaborations with Eyevensys,GlucoModicum and Credence MedSystems accelerate delivery of technology breakthroughs and next-gen products
HUDSON,Wis.,Oct. 20,2021 --Phillips-Medisize,a Molex company and leader in the design and manufacture of drug delivery,diagnostic and MedTech devices,announces the expansion of its global manufacturing footprint,along with extended product design,development and manufacturing capabilities to streamline the delivery of game-changing products and solutions. The company's global reach now encompasses 36 world-class facilities with scalable,end-to-end capabilities tailored to help customers bring groundbreaking products to market quickly and efficiently from anywhere in the world.
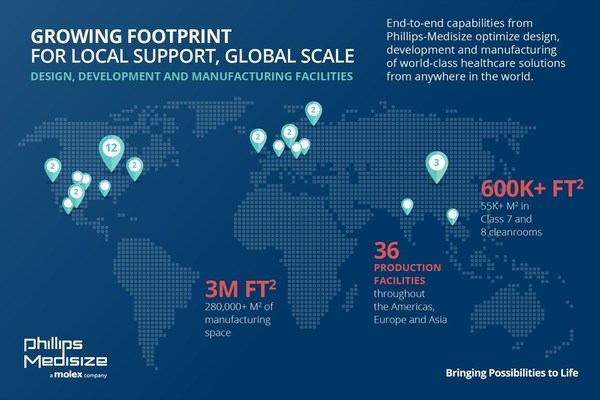
Growing footprint of Phillips-Medisize’s 36 world-class facilities around the globe.
"As the preferred partner for leading global customers,we continually invest in new talent and technologies that increase the capacity and capabilities of Phillips-Medisize across the entire value chain," said Paul Chaffin,president,Medical and Pharmaceutical Solutions,Molex. "Our expanded global reach and resources will enable us to solve complex development and manufacturing challenges while meeting escalating customer demands for more localized production,supply chain management and accelerated go-to-market strategies."
Growing Footprint for Local Support,Global Scale
To address growing production demands from customers in continental Europe,Phillips-Medisize is building a state-of-the-art medical manufacturing facility in Katowice,Poland. Slated to open in 2022,the site will complement production sites and innovation centers in Asia,Europe,India,Mexico and North America. Phillips-Medisize also is expanding production capacity in Suzhou,China to serve both global and regional pharmaceutical and MedTech customers. Additionally,the transformation of an existing Molex production facility in Little Rock,Ark.,is underway,enabling Phillips-Medisize to keep pace with ever-increasing requirements in the U.S. for high-volume,diagnostic device manufacturing.
Once these expansions are complete,Phillips-Medisize will offer nearly three million square feet/280,000 square meters of country- and region-specific manufacturing space and R&D capabilities worldwide. The company also will support 600,000 square feet/55,000 square meters of Class 7 and 8 cleanrooms,which complement existing tool building sites as well as global quality and regulatory systems. In 2020,Phillips-Medisize completed a new facility in St. Croix Meadows,Wisc.,with 285,000 square feet/26,000 square meters supporting production of molded components for medical diagnostic customers requiring high-volume assembly and packaging of regulated products. The facility also includes a nearly 64,000 square-foot/6,000 square-meter ISO 14644-1 Class 8 cleanroom.
Extending End-to-End Capabilities
As part of its one-stop shop of medical-device manufacturing solutions,Phillips-Medisize focuses on design for manufacturability and assembly excellence. Proven front-end innovation,human-factors engineering,and quality are integrated with regulatory adherence to reduce go-to-market risk and cost. Expanded capabilities in complex molding,drug and reagent handling,as well as final packaging and serialization,enable customers to consolidate global supply chains while optimizing go-to-market strategies.
Phillips-Medisize's combined expertise in plastics,metals,electronics and connectivity ease the development of different solutions,including combination devices such as needle-based injection systems and wearable injection technologies. An unwavering commitment to rigorous quality management is reinforced by dedicated New Product Introduction (NPI) teams at every site. Strategic investments in talent acquisition to support Phillips-Medisize's continued growth are expected to add at least 1,000 employees worldwide over the next couple of years.
Optimizing Industry-Leading Collaborations
A strong track record of collaboration ensures Phillips-Medisize is at the forefront of the latest advancements in drug delivery,diagnostic and MedTech devices. Today,Credence MedSystems announced a strategic initiative with Phillips-Medisize that includes ramping production of the Credence Companion®and Dual Chamber Reconstitution Systems at the Phillips-Medisize Letterkenny,Ireland site and the new facility in Poland.
"We're excited to leverage Phillips-Medisize's world-class manufacturing as we scale toward high-volume automation at the new state-of-the-art facility in Poland," said Jeff Tillack,COO at Credence MedSystems. "The opportunity to scale production in close proximity to our operations and European customers will accelerate market delivery of our innovative solutions to meet the needs of our pharma customers and their end-users."
In September,GlucoModicum entered into a design and development program with Phillips-Medisize for the rapid scale-up of manufacturing for its Talisman needle-free continuous glucose monitor,which uses unique magnetohydrodynamic (MHD) technology. Also last month,Paris-based Eyevensys announced an engagement with Phillips-Medisize to optimize the design,development and high-volume manufacturing of its ocular device component that powers the delivery of novel gene therapies for treating various eye diseases.
Phillips-Medisize Brings Possibilities to Life
Phillips-Medisize,a Molex company,brings decades of innovation to leading healthcare and life science companies to develop groundbreaking solutions that help people live healthier,more productive lives. On average,the company commercializes 50 new products a year for customers,including the first-to-market FDA-registered drug-delivery device utilizing a connected health system. Molex brings decades of experience in advanced electronics,connectivity and sensor technologies to help transform medical and pharmaceutical solutions.
About Molex
Molex is a global electronics leader committed to making the world a better,more-connected place.With presence in more than 40 countries,Molex enables transformative technology innovation in the automotive,data center,industrial automation,healthcare,5G,cloud and consumer device industries. Through trusted customer and industry relationships,unrivaled engineering expertise,and product quality and reliability,Molex realizes the infinite potential ofCreating Connections for Life. For more information,visit www.molex.com.