SQN Clinical Launches the Most Advanced Real-time Analytics Tool for Clinical Research
DISS,England,July 17,2018 --
SQN Clinical launches SQN Health Analytics,providing clinical professionals with a clear picture of the entire trial process and all collected data,whenever and wherever they need it.
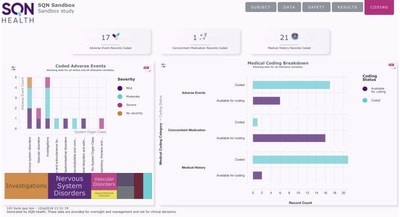
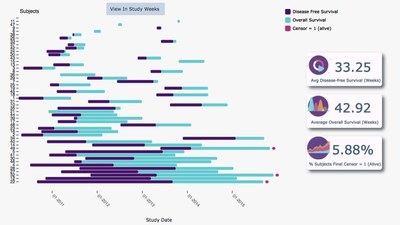
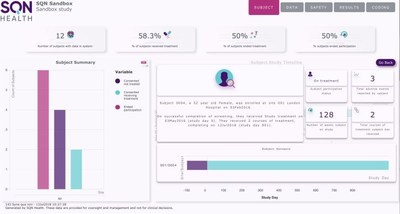
With high-level summary information,advanced filtering options and the ability to drill down to individual patient records,SQN Health Analytics marks the end of manual,complex and time-consuming data exploration. Sponsors now have greater control,identifying potential issues before they arise and allowing for more robust trial management and in-trial decision making.
This cloud-based suite is the latest addition to SQN Health,the electronic data capture (EDC) system that incorporates the recently launched,patient-centric, electronic patient reported outcomes (ePRO) app.
"Reliable data is the cornerstone of clinical research and thanks to recent advances in technology we can now collect more meaningful and usable information than ever before," explains Tony Rees,Director at SQN Clinical. "The challenge is to maximise the use of data to better support the clinical trial process and patient oversight. Our advanced data analytics,incorporating artificial intelligence (AI) components,now delivers some of the most advanced project and clinical oversight capability within the industry."
Sponsors have to demonstrate effective oversight of their clinical trials and increasingly have the need to explore data relationships and various clinical parameters. SQN Clinical Analytics delivers this in real-time allowing for data review,patient profiling and exploratory analysis in dynamic ways not previously seen. This adds significant value to the management of patients and trials and is of particular benefit in supporting data safety monitoring boards (DSMB).
Responsive and flexible analytics that evolve with each trial
Joseph Jameson,Data Analyst at SQN,explains the concept behind SQN Health Analytics: "We recognised our clients' needs to improve clinical oversight and efficiency within clinical research. They need an analytics suite that is flexible,responsive and reflects the real-time fluctuations and unique evolution of their particular trials. Each trial is unique and we are now able to rapidly deploy bespoke analytics reports that specifically meet their needs.
"We can display any trial data in a clear and uncluttered way,real-time,anytime and anywhere. Reports and snapshots can still be generated for any point in the trial,providing reliable data for review meetings and to support decision making. The system allows for comparative analytics between or within trials,providing the opportunity to explore relationships and patterns that weren't easy to see before.
"Our analytics suite is fully integrated with our EDC and ePRO systems providing a seamless data ecosystem. It gives sponsors access to information according to their role and the predefined access rights given to them. Senior teams can view high-level data for oversight management,comparing multiple sites and tandem trials. Dashboards with user-defined KPIs provide an instant snapshot of any element of the trial,and the data can be sorted and filtered in multiple ways to show any combination of factors.
"By cross-comparing data from both the EDC and ePRO systems we can clearly see the impact of patient-reported outcomes on clinical trial data,supporting the Food and Drugs Administration (FDA) focus on the importance of this information.
"Our analytics does not use generic software adapted from another unrelated industry but has been specifically designed and built to support the complexity of clinical trial designs and their unique underlying data needs. It brings the data to life in a way not previously seen and includes a dynamic option to export selected data for further review,analysis and reporting.
Keeping trials on track
"This development adds value for our clients and represents a significant step forward in the management of clinical trial data," states Rees. "SQN Health Analytics will enhance the ways in which our clients interact with and use their data and. With their input,we are already developing innovative new ways in which we can extend the analytics functionality."
"Future phases will include machine learning and further artificial intelligence to provide predictive modelling and forecasting capabilities. Our aim is to help professionals anticipate issues and events before they arise and enable them to take proactive steps to keep their trials on track.
"SQN Health Analytics gives clinical teams the ability to interrogate trail audit data in a way not previously possible,delivering a new standard in supporting trial integrity. Our goal is to help pharmaceutical and biotech companies to get their products to market in a safer,more effective way. We do that by giving them the insight and control they've long been looking for."