Agilis RoboticsTM Completes New Round of Live Animal Trials with Miniaturized Robotic Instruments for Endoscopic Surgery
HONG KONG,Jan. 17,2023 -- Agilis RoboticsTM (Agilis),a leading developer of flexible robotic instruments that support endoscopic surgery through natural orifices of the body (e.g. along the gastrointestinal tract and in the bladder),has recently completed the second round of live animal testing using its proprietary robot. Dr. Jason Y.K. Chan,the co-founder of Agilis RoboticsTM,conducted the test with the firm's second-generation device on a live pig specimen and successfully removed artificial tumors using the en-bloc resection of bladder tumor (ERBT) technique. The test results met expectations,demonstrating promising results for the accuracy,safety,and efficacy of the firm's medical robotic system.
Drawing on the advice of surgeons in earlier experiments,the research team has made several technical improvements to the original system that increased the efficiency of the surgery.
The 2nd generation prototype system has been optimized for more streamlined surgical control
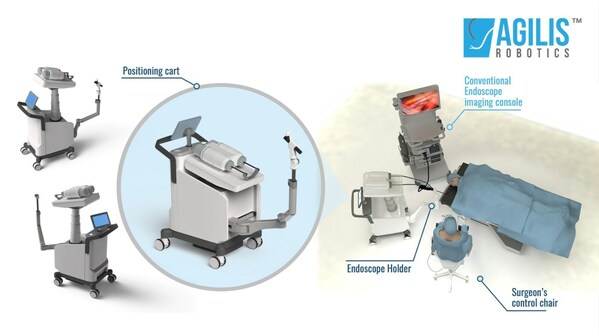
The Agilis RoboticsTMsystem is a surgical robotic solution designed for endoscopic surgery. The system features a set of flexible surgical robotic instruments,a positioning cart and a surgeon's control chair. The flexible robotic instruments,with a diameter of less than 2.8 mm,can grip and cut away tumor tissue to remove it in its entirety.
The second-generation prototype system used in this test includes several improvements over the original system. With a smaller footprint,the updated positioning cart is 50% smaller,making it highly compact and easily positioned in the operating room.
Second,the newly developed endoscope holder offers the surgeon great flexibility for positioning the endoscope and robotic instruments. During operation,the surgeon can move the endoscope with ease using just one hand in thanks to the holder's sensor. The holder includes an automatic locking mechanism that prevents the unit from moving around freely,streamlining the surgeon's work and improving the procedure's safety.
The surgical tools have also been improved in this prototype iteration,allowing the surgeon to visualize the surgical field beyond the instruments more clearly and increase its load capacity to meet more demanding requirements. The electrosurgical knife instrument was also improved to enable bipolar diathermy,which substantially enhances tissue cutting effectiveness in bladder tumor resection. The most notable improvements are made to the controls and mechanical transmission in terms of precision and reduced control latency. This gives the surgeon a more intuitive and responsive control experience compared with manual operation. In addition to the controls,the team has also made substantial improvements to the system's ergonomic design and training simulator.
Next,the company will test the system in animals for upper and lower gastrointestinal (GI) endoscopic submucosal dissection (ESD). This will be a major milestone for the company,demonstrating their potential within the sizeable market for GI diseases.
Agilis RoboticsTMinvited prominent gastroenterology and hepatology specialist Dr. Peter Chan Chun Wing to evaluate the system before its entry into the digestive tract market. Dr. Chan has almost 20 years of medical specialty practice,focusing on endoscopy and GI surgery. Dr. Chan is listed as a colonoscopy provider to the Colorectal Cancer Screening Programme of the Hong Kong Department of Health,offering pre-screening advice and colonoscopies for eligible individuals.
As the first internal medicine physician to use the system,Dr. Chan not only carried out ev-vivo tissue resection tests but also offered guidance on the system's potential for the GI market. He foresees the system's accessibility in general endoscopy clinics and centers,which is crucial to making the system widely adopted throughout healthcare institutions.
Agilis RoboticsTM is challenging convention with its unmatched intuitiveness as minimally invasive endoscopic procedures are rapidly evolving
A large number of new minimally invasive endoscopic techniques,including EMR (endoscopic mucosal resection) and ESD,have emerged to combat the increased detection of submucosal tumors,early cancers,and precancerous lesions in the digestive tract,which is a positive outcome due to rise in health awareness among the general population. By eliminating external incisions and promoting quick recovery,the practical use of these techniques has improved patient outcomes and decreased discomfort.
Endoscopic minimally-invasive interventional techniqueshave evolved quickly in recent years and are becoming more important in the treatment of digestive disorders.
Interventional procedures are more popular with patients as they are less invasive and require a shorter hospital stay. By enabling complete excision of diseased tissue while maintaining the normal function of the digestive tract,the approach can reduce the risk of post-operative complications such as infection associated with surgical operations (e.g.,major surgery such as open-heart and open-abdomen).
Patients who received endoscopic surgery recover quickly and stay in the hospital for a much shorter time—usually 2 to 3 days or less—before being discharged. The cost of treatment is only 1/3 to 1/2 of a general surgery. Industry insiders have said that although general surgery costs between 20,000 and 40,000 yuan,endoscopic minimally-invasive procedures can be managed for as little as 20,000 yuan.
In contrast to previous prototype tests,the company invited laypeople with no medical professionals to observe. They briefly used a virtual training simulator and then carried out tissue dissection in an ex-vivo tissue model.
Participants commented,"The Agilis surgical robot is remarkable,even for an unskilled learner,as it can already remove artificial tumors from ex-vivo tissue after around 20 minutes of training with the simulator." The visitors claimed that the technology is simple to use and requires little training. Doctors skilled in endoscopic procedures must be able to pick up the skills quickly. They also expressed optimism about the device's commercialization potential.
The Agilis system also aims to support training using artificial intelligence (AI). Empirical endoscopic imaging is used in the AI system for deep learning to enhance the operator's surgical training. The learning curve for performing en-bloc tumor resection should be greatly reduced,as compared to manual ESD operations which are only performed by surgeons with extensive experience.
A US$100 million firm focused on creating robotic equipment for endoscopic surgery
Surgical robots in laparoscopy,orthopedic surgery,neurosurgery,vascular intervention,and other specialties are now popular topics in China. Businesses,however,need to think about how to break through the bottleneck of technological innovation in light of the highly competitive market.
Several challenges have been identified in the course of developing robots for endoscopic surgery. For example,general endoscope working channels (2.8–3.7mm in diameter),require considerable flexibility to adapt to the changing shape as the endoscope travels through the narrow passageways within human body,resulting in high demands for the size and ergonomic design of the overall system.
Agilis RoboticsTM is the first company to develop such a robotic system to overcome these challenges. The company has created a surgical robotic device that can seamlessly integrate with existing surgical procedures,notably for the GI and urological systems,to boost surgical efficiency and reduce the learning curve for endoscopic surgery. The firm's robotic instruments are totally flexible and has achieved a range of technological achievements.
As a kind of natural orifice transluminal endoscopic surgery (NOTES),endoscopic surgery is distinct from laparoscopy and requires a notably different set of technological skills. Major global players such as Johnson & Johnson,Intuitive Surgical,and Medtronic have developed their own surgical robotic systems,however none are working with the approach of utilizingconventional endoscopes and their working channels. Presently,R&D for NOTES is focused on the bronchial sector in China,none of which has received approval for use in the country as well.
According to the US-based business consulting firm Frost & Sullivan,the global NOTES robot market is expected to grow at a CAGR of 88.2% from 2016 to 2026,maintaining significant growth,and is on track to reach 12.53 billion yuan by 2026 from 457 million yuan in 2020.
There is still room for growth in the market for endoscopic surgery robots for the urology and digestive systems. As the first Chinese business to build an endoscopic surgery robot,Agilis RoboticsTM has a head start on the competition in the market with a product that is already taking shape and has plans for pre-clinical testing.
The second-generation prototype used has optimized the structure and integration of the firm's flexible robot instruments in many ways. "Our next step is to improve the robotics control and console's design and control features in a move to further improve surgical accuracy and efficiency," Agilis RoboticsTM revealed. The company is planning for next iteration prototype testing,and expects to complete the first human trial in the coming two years.
In June 2022,Agilis RoboticsTM closed its Series A fundraising. Joe Hui,co-founder and CFO,said that the advanced prototype will be delivered to The University of Hong Kong-Shenzhen Hospital in the second quarter of this year for additional testing. The company will also wrap up its NMPA testing in the third quarter of 2023 and then start a PRE-B round of funding with a minimum investment of US$10 million. The COVID-19 outbreak last year prevented investors from visiting to Hong Kong for test and experience the system in Series A roadshows. Now,in an effort to entice investors,the company has established a special Series A+ of US$5 million.