Agilis Robotics conducts first live animal trial for GI indication, targeting 510(k) clearance by 2025 to transform endoscopic surgery
HONG KONG,April 19,2023 --Agilis Robotics,an innovative spin-off company from The University of Hong Kong,is making waves in the realm of endoscopic surgery with its flexible robotic arms. Their creation showcases highly miniaturized and flexible endoscopic surgical robotic arms,making it a game-changer for endoluminal surgery for medical practitioners and patients alike.
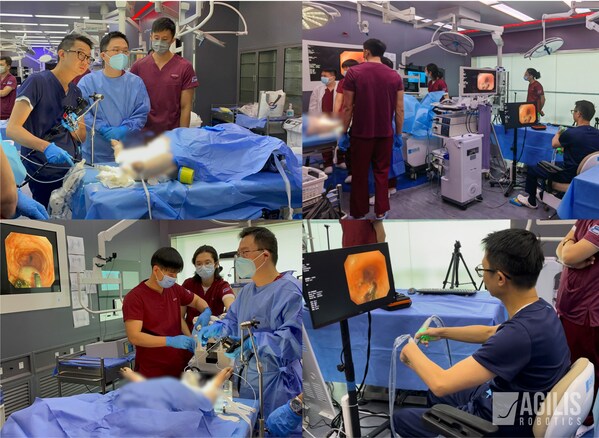
Live swine trial in The University of Hong Kong-ShenzhenHospital
The system by Agilis Robotics offers a novel combination of features that set it apart from conventional surgical robotic systems. With a remarkably compact design and intuitive interface that involves minimal learning curve,this advanced surgical system is poised to transform the field of minimally invasive surgeries.
The most noteworthy characteristic of the system is its unparalleled flexibility. With 5 degrees of freedom per arm and a totally flexible body,it allows surgeons to execute complex procedures of tissue resection with great precision and ease inside natural orifices. Along with its intuitive control interface and the aid of artificial intelligence,this exceptional maneuverability is invaluable in endoscopic surgery,where dexterous manipulation of surgical tools is highly challenging without robot assistance.
Another standout quality of the system by Agilis Robotics is its affordability. The system consists of a control console with disposable robot arms and accessories. Due to being directly compatible with conventional rigid and flexible endoscopes already in use by hospitals,the system can be low-cost and compact. No large upfront investment in operating theatre design and machinery procurement is required. By providing a low-cost option,Agilis Robotics will make robot-assisted endoscopic surgeries more accessible to hospitals and clinics globally,improving patient outcomes and reducing healthcare costs.
Agilis Robotics has recently showcased the system's capabilities in a trial performed on a live pig subject at The University of Hong Kong-Shenzhen Hospital in March. Dr Joe FAN King Man led the trial and successfully performed endoscopic submucosal dissection (ESD) in the upper and lower gastrointestinal tract with the robotic system.
An additional endoscopic ex-vivo trial on trachea tissue was also performed. Tissue from the trachea was successfully dissected,further demonstrating the versatility and adaptability of the system in various surgical scenarios.
With these promising results under its belt,Agilis Robotics is setting its sights on obtaining FDA 510(k) clearance by 2025. This clearance would allow the company to market its robotic system,and further realize its ambition to make impactful change in endoscopic surgery.