ISCT 2023: Late-breaking SCG101 Data Show 100% HBsAg+ Hepatocyte Eradication and 74.5% HCC Tumour Reduction With Single Dose HBsAg-specific TCR-T Cell Therapy
SINGAPORE,June 5,2023 -- SCG Cell Therapy Pte Ltd (SCG),a clinical-stage biotechnology company developing novel immunotherapies for infectious diseases and their associated cancers,presented late-breaking data from its first-in-class autologous HBsAg-specificT-Cell receptor-engineered T Cell (TCR-T) Therapy– SCG101 – at the International Society for Cell & Gene Therapy (ISCT) conference in Paris,France.
SCG101 showed significant antitumor activities with a tumour reduction of 74.5% per mRECIST after single dose SCG101 monotherapy treatment. A durable tumour response of >6.9 months was reported by data cut-off.
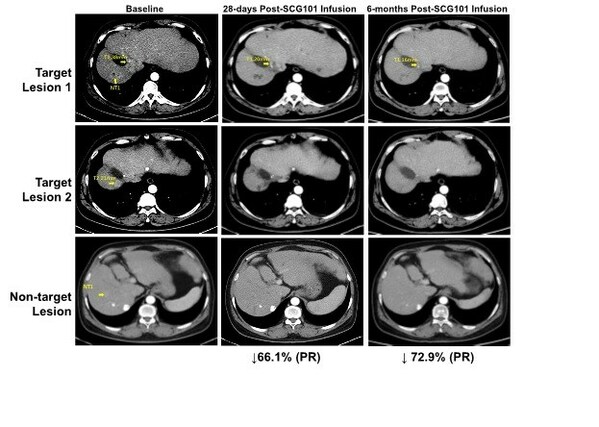
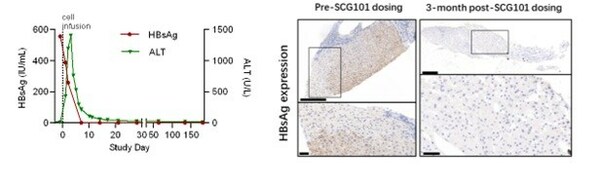
Pre-treatment and post-treatment biopsies showed 100% eradication of HBsAg+ hepatocytesin the liver. Serum HBsAg reduced from 557.96 IU/mL at baseline to 1.3 IU/mL on Day 7 and further reduced to 0.08 IU/mL (3.83 log reduction) by Day 28 after SCG101 infusion.
Hepatitis B Virus (HBV) infection is a leading cause of liver cancer and accounts for 50%-80% of hepatocellular carcinoma cases worldwide.[1] HBV DNA integrates into the host genome and leads to genetic instability of the host cell and epigenetic remodelling of host DNA,resulting in abnormal expression of oncogenes and HBV antigens.[2] SCG101 can specifically target epitope peptide derived from Hepatitis B Surface Antigen (HBsAg) presented on HBV-HCC tumour cells,premalignant cells with HBV-DNA integration and HBV infected cells,triggering cytolytic and non-cytolytic mechanisms to eliminate tumour cells and HBV-infected cells.
"Tumour microenvironment plays a critical role in limiting the response to cancer immunotherapy. Targeting and clearance of HBsAg+ hepatocytes and HBV integrated premalignant dysfunctional cells would significantly contribute to SCG101 T cell stimulation and expansion before entering the hostile tumour microenvironment as well as modulating the immunosuppressive environment in the liver",said Dr. Zhang Ke,Chief Scientific Officer of SCG Cell Therapy. "This unique property of SCG101 can effectively overcome a common challenge of T cell therapy,particularly for solid tumours". He added.
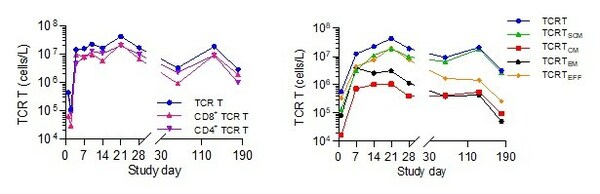
Through biomarker analysis,SCG101 demonstrated unique CD8/CD4 dual functionality. After SCG101 infusion,well-balanced of CD8:CD4 ratio were observed demonstrating strong immune response triggered by SCG101. Persistent and high ratio of stem cell-like memory T cells (Tscm) correlated with the long-term T cell proliferation supports prolonged T cell functions and sustained antitumour and antiviral responses.
"These exciting new data being presented at ISCT continue to reinforce the potential for viral antigen specific TCR-T cell therapy products to effectively treat infection-associated solid tumour indications like HBV-related hepatocellular carcinoma," said Frank Wang,Chief Executive Officer of SCG Cell Therapy. "Thus far,SCG101 has shown to produce robust and durable anti-tumour and antiviral responses and we look forward to providing additional updates on this program as the clinical trial progresses."
About SCG101
SCG101,an autologous T-cell receptor (TCR) T cell therapy,is an investigational cell therapy product targeting specific epitopes of hepatitis B surface antigen (HBsAg). Utilizing SCG's proprietary GianTTM technology,high affinity and high avidity natural TCRs can be identified against intracellular antigens expressed through major histocompatibility complex (MHC) in solid tumours. Preclinical and clinical studies of SCG101 demonstrated tumour inhibition and HBV cccDNA eradication. SCG101 was granted clinical trial approvals by the U.S Food and Drug Administration (FDA),China National Medical Products Administration (NMPA) and Singapore Health Science Authority (HSA) for patient with HBV-related HCC. A Phase 1/2 clinical trial evaluating SCG101 is ongoing.
About Hepatocellular carcinoma
Hepatocellular carcinoma (HCC) is the most common type of liver cancer. It is estimated more than 905,000 new cases of liver cancer and more than 830,100 deaths from the disease globally in 2020,making it one of the leading causes of cancer deaths around the world.[3] Chronic hepatitis B virus (HBV) infection accounts for at least 50% of cases of HCC worldwide.[1] HCC is typically diagnosed at an advanced stage and is associated with a poor prognosis. The five-year survival rate of less than 15%.
About SCG Cell Therapy
SCG is a leading biotechnology company focusing on the development of novel immunotherapies in infections and its associated cancers. The company targets the most common cancer-causing infections: helicobacter pylori,human papillomavirus,and hepatitis B,and develops a broad and unique pipeline of T cell therapies,antibodies,and therapeutic vaccines against infections and to prevent and cure its associated cancers. Established and headquartered in Singapore,SCG combines regional advantages in Singapore,China and Germany,covering the entire value chain from innovative drug research and discovery,manufacturing,clinical development and commercialization. For more information about SCG,please visit us at www.scgcell.com.
[1] Y,Xie. (2017). Hepatitis B virus-associated hepatocellular carcinoma. Advances in experimental medicine and biology. https://pubmed.ncbi.nlm.nih.gov/29052129/
[2] Jiang,Y.,Han,Q.,Zhao,H.,& Zhang,J. (2021,May 20). The mechanisms of HBV-induced hepatocellular carcinoma. Journal of hepatocellular carcinoma. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8147889/
[3] Liver cancer statistics: World cancer research fund international. WCRF International. (2022,April 14). https://www.wcrf.org/cancer-trends/liver-cancer-statistics/