BioCity announces the first patient dosed with its first-in-class CDH3-targeting ADC BC3195 in a Phase 1 Trial
WUXI,China,July 20,2023 -- BioCity Biopharma,a clinical-stage biopharmaceutical company committed to developing novel and highly differentiated,modality-independent therapeutics for cancer and autoimmune disorders,today announced the dosing of the first patient in a Phase 1a/1b clinical trial of its first-in-class CDH3 antibody drug conjugate (ADC),BC3195 in China. The study will assess the safety,tolerability,pharmacokinetics,and preliminary efficacy of BC3195 in patients with locally advanced or metastatic solid tumors.
BC3195 is a novel ADC targeting CDH3/P-cadherin,a calcium-dependent cell-cell adhesion glycoprotein highly expressed in a variety of malignancies,including lung,breast,gastric,ovarian,colorectal,and pancreatic cancers. CDH3 expression is low or undetectable in normal tissues,making it an ideal target for anticancer therapies,particularly ADCs.
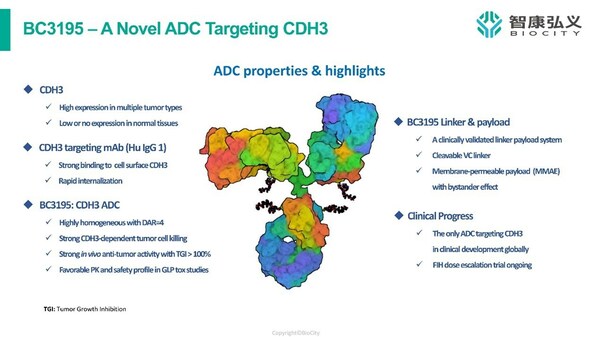
The properties and highlights of BC3195
Currently,BC3195 is the only ADC targeting CDH3 in clinical development globally. In preclinical studies,BC3195 binds to cell surface CDH3 with strong affinity and is efficiently internalized. BC3195 is designed with a clinically validated,cleavable linker and payload (vc-MMAE) allowing for the destruction of targeted tumor cells as well as surrounding cells which is known as the bystander effect. In animal models,BC3195 demonstrated a favorable safety profile and strong antitumor activity with tumor growth inhibition of ≥100%.
In addition to clinical trials being conducted in China,BC3195 has an active US IND. BioCity Biopharma expects to obtain preliminary clinical data on BC3195 by the end of 2023 from clinical trials in China as well as in the US.
About BioCity
Founded in December 2017,BioCity is a clinical-stage biopharmaceutical company committed to developing novel and highly differentiated,modality-independent therapeutics for cancer and autoimmune disorders including chronic kidney diseases (CKD). The company has established a pipeline of more than 10 innovative drug candidates including small molecules,monoclonal and bispecific antibodies as well as antibody-drug conjugates (ADCs).
Currently,BioCity Biopharma has 6 oncology projects in Phase 1 development,including the first-in-Class CDH3-targeting ADC,and agents targeting the DNA damage response (DDR) pathway via a WEE1 and an ATR inhibitor,and agents targeting the immune system with a T cell engager (CD3XEGFR BsAb),an immune checkpoint inhibitor (TIM-3 mAb),and a T cell activator (4-1BB mAb). In addition,anendothelin A(ETA)-receptor selective antagonist designed for CKD has entered phase 2 development.
For more information,please visitwww.biocitypharma.com
Contact:
BD@biocitypharma.com
IR@biocitypharma.com