Rapid Medical Receives CE Mark Approval for TIGERTRIEVER 13
YOKNEAM,Israel,Aug. 1,2018 --
TheTIGERTRIEVER13 is thesmallest profile stentriever,designed tosafelyretrieve clots fromintracranialvessels of 1mm-2.5mm.
Rapid Medical,a company focused on the development of next generation neurovascular devices,has announced that it has received CE Mark approval for the TIGERTRIEVER 13. In addition,first patients have been treated successfully with the device.
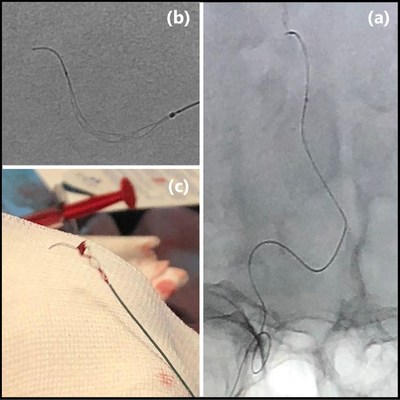
TIGERTRIEVER,the first-ever adjustable,fully visible clot retriever is designed to treat ischemic stroke patients. About 1,500 patients have been successfully treated with the TIGERTRIEVER so far,the TIGERTRIEVER 13 is the newest addition of the Tigertriever family. Its default profile is 83% smaller than any other device on the market and it is delivered through a neurovascular microcatheter with a soft distal outer diameter of 1.3Fr. It is designed to recanalize intracranial vessels of 1mm - 2.5mm. These medium vessel occlusions (MVO) may account for up to 30% of ischemic stroke patients and cannot be treated by any other device on the market.
"TIGERTRIEVER 13 is a very important addition to the ischemic stroke device market," said Prof. Rene Chapot,Germany. "For the first time ever,we have a tool that is dedicated to more distal occlusions. These occlusions can have a dramatic disabling effect on patients and until now there was little to be done for them. Using the Tigertriever 13 we were able to retrieve clots from an MVO that was not treatable until now."
Dr. Jeffrey Saver,professor of neurology and director of the comprehensive stroke center at the David Geffen School of Medicine of UCLA,commented on the first clinical experience with the TIGERTRIEVER 13: "We know that endovascular therapy is the best option for large vessel occlusions. The TIGERTRIEVER 13 will further extend this powerful treatment for acute ischemic stroke to patients with medium vessel occlusions (MVOs)."
Rapid Medical will launch the TIGERTRIEVER 13 inEuropeduring Q3 2018.
About Rapid Medical
Rapid Medical is developing game-changing devices for endovascular treatments. Rapid Medical is the maker of TIGERTRIEVER family,fully visible family of clot retriever that are designed to treat ischemic stroke patients,and the COMANECI,the first-ever adjustable aneurysm remodeling mesh. TIGERTRIEVER and COMANECI are CE marked for use in Europe and are also available for use in Canada and India. More information is available athttp://www.rapid-medical.com
Contact:
Ronen Eckhouse
+972-72-2503331
ronen@rapid-medical.com