ZEISS showcases next-level eye care digitalization at AAO 2019

SAN FRANCISCO,Oct. 12,2019 -- At the Annual Meeting of the American Academy of Ophthalmology (AAO) in San Francisco,ZEISS Medical Technology Segment will present the latest in digital eye care innovation and high-resolution imaging from October 12 – 15,2019. With the vision and the expertise to bring digital technology to the next level,ZEISS is helping doctors provide their patients with transformational care.
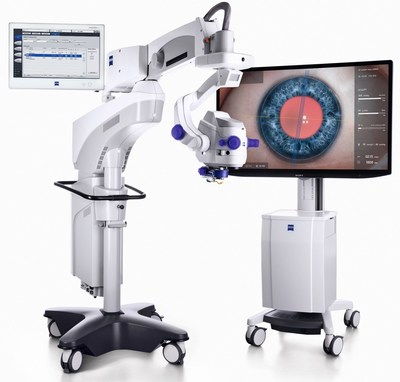
ZEISS ARTEVO 800
"ZEISS has set the pace in digital advancements,collaborating with leading doctors and scientists around the world with integrated,data-driven solutions," says Dr. Ludwin Monz,President and CEO of Carl Zeiss Meditec. "We're excited to be here at AAO,demonstrating our commitment to providing customers with innovative platforms,so they can provide their patients with digital care every step of the way."
ZEISS redefines digital workflow with integrated diagnostic solutions for improved clinical efficiency and patient care
The ZEISS Integrated Diagnostic Imaging platform,a software-driven,multi-modality solution that gathers,combines and associates data from different diagnostics devices,allows doctors to individualize treatment to their patients' specific needs,creating a digital workflow that enhances treatment capabilities and expands the level of care.
With the onset of new technologies and vast amounts of information that doctors are required to absorb,fast and high-quality diagnostics are critical for creating customized treatment regimens enabling clinicians to spend more time with their patients. With the Integrated Diagnostic Imaging platform,ZEISS creates the ideal digital workflow using solutions like the CIRRUS® 6000 and the ZEISS Glaucoma Workplace.
The ZEISS CIRRUS 6000,the next generation,ultra-fast 100kHz system,promises to dramatically elevate the efficiency of busy,advanced care practices. At 100,000 scans per second,image acquisition is extremely fast. The ZEISS CIRRUS 6000 OCT scans are 270 percent faster than prior CIRRUS generations,43 percent faster than the ZEISS CIRRUS 5000,and ZEISS AngioPlex and OCT cube scans can be acquired in as little as 0.4 seconds. Seamless transfer of raw patient data from previous generations makes it easy to analyze and preserve old,new,and future patient data.
The ZEISS Glaucoma Workplace integrates perimetry and OCT to help doctors streamline complex analysis. The new version of the ZEISS Glaucoma Workplace features a new Progression Summary report with "exception-based" alerts that automatically display statistically significant change in critical measurements such as Visual Field,RNFL,GCL,C/D Ratio and IOP change. The ZEISS Glaucoma Workplace helps deliver a new level of efficiency for glaucoma data analysis.
In addition to the ZEISS Integrated Diagnostic Imaging platform,the ZEISS SL 800 offers further innovative and ergonomic workflow improvements for a more straight-forward,uninterrupted eye exam that frees up one of the doctor's hands. Equipped with AutoView,a motorized two-button mechanism for changing magnification right next to the joystick,an electronic QuickStop brake,and the unique VarioLight feature that combines all advantages of LED and halogen illumination characteristics,ZEISS SL 800 facilitates more focus on doctor-to-patient interaction and sets a new standard in slit lamps.
ZEISS ARTEVO 800 – a new digital era in surgical visualization
The ARTEVO® 800,the first digital microscope in ophthalmic surgery,is now commercially available in the U.S. The ZEISS ARTEVO 800 was developed with surgeons for surgeons and includes integrated digital optics for optimized digital visualization and cloud connectivity to the EQ Workplace® for remote access to patient data and images.
"I am thrilled with the digital microscope!" said Sri Ganesh,MD,Chairman and Managing Director of Nethradhama Super Speciality Eye Hospital,Bangalore,India. "The visualization is amazing - I don't miss my oculars at all."
"We are excited to offer surgeons an improved surgical experience with the commercially-available ZEISS ARTEVO 800," said Jim Mazzo,Global President Ophthalmic Devices at Carl Zeiss Meditec. "With revolutionizing visualization,information,comfort and workflow in the operating room,the ARTEVO® 800 is going to change the future of surgical care."
The ZEISS ARTEVO 800 brings the proven strengths of ZEISS optic engineering together with the countless capabilities of digital imaging and embeds that into the workflow of the company's Cataract Suite with a full range of diagnostic and surgical products. With ZEISS ARTEVO 800,ZEISS is entering a new era of digital ophthalmic visualization,allowing customers to see more.
Additionally,ZEISS is expanding its data management system FORUM® with the ZEISS EQ Workplace,allowing customers to remotely plan IOL surgery to streamline their refractive cataract workflow. Along with ZEISS Veracity Surgical,ZEISS offers digital workflow solutions for a wide variety of customer preferences and infrastructure requirements.
ZEISS leads the way with new refractive technologies in the U.S.
ZEISS demonstrates its commitment to the U.S. refractive market with the MEL 90 clinical trial across eight investigational sites. At a time when most companies are focused on modifying existing platforms,ZEISS is leading the way by bringing new refractive technologies to the U.S. market,starting with the VisuMax® laser for SMILE® and continuing with the MEL 90.
With more than 2 million Small Incision Lenticule Extraction (SMILE®) treatments to date,ZEISS continues its leadership in laser vision correction with SMILE® currently used by more than 2,000 surgeons across more than 70 countries worldwide. With FDA approval late last year,ZEISS expanded its Myopia treatment to patients with Astigmatism and has seen the volume of treatments with SMILE® more than double in the U.S. compared to last year.
"As one of the first surgeons in California to perform the SMILE®-based procedure I have since seen a drastic improvement in patient care," said Jay Bansal,of the LaserVue Eye Center in the San Francisco Bay Area. "The minimally-invasive nature of the procedure improves the overall patient experience and the solution set up makes it more efficient and economical for my practice."
SMILE® supports a minimally-invasive corneal refractive procedure performed by using the VisuMax® femtosecond laser to create a thin disc-shaped lenticule within the cornea,which is then removed through a small incision,thereby achieving the desired vision correction. SMILE® requires only one laser to perform the entire solution leaving the outer corneal layer largely intact,thus potentially contributing to the cornea's biomechanical and refractive stability after surgery.
As some indications in refractive surgery still require treatment by an ablative laser,ZEISS has initiated a clinical trial for the MEL 90 Excimer Laser. With IDE approval received earlier this year,first treatments have commenced prior to AAO. "We are thrilled to be starting this study now and to eventually support an approval that will complement our surgical toolbox beyond the indications of the SMILE® -based procedure," said Jon Dishler,of Dishler Laser in Denver,Colorado,and Medical Monitor for the MEL 90 clinical trial.
ZEISS will showcase the latest solutions for digital eye care in Booth # 5669 in North Hall at the Annual Meeting of the American Academy of Ophthalmology (AAO) from October 12-15,2019.
For more information,visit www.zeiss.com/meditec/
Not all products,services or offers are approved or offered in every market and approved labeling and instructions may vary from one country to another. For country specific product information,see the appropriate country website. Product specifications are subject to change in design and scope of delivery as a result of ongoing technical development.
Brief profile
Carl Zeiss Meditec AG (ISIN: DE 0005313704),which is listed on MDAX and TecDax of the German stock exchange,is one of the world's leading medical technology companies. The Company supplies innovative technologies and application-oriented solutions designed to help doctors improve the quality of life of their patients. The Company offers complete solutions,including implants and consumables,to diagnose and treat eye diseases. The Company creates innovative visualization solutions in the field of microsurgery.
With approximately 3,050 employees worldwide,the Group generated revenue of €1,280.9m in fiscal year 2017/18 (to 30 September).
The Group's head office is located in Jena,Germany,and it has subsidiaries in Germany and abroad; more than 50 percent of its employees are based in the USA,Japan,Spain and France. The Center for Application and Research (CARIn) in Bangalore,India and the Carl Zeiss Innovations Center for Research and Development in Shanghai,China,strengthen the Company's presence in these rapidly developing economies. Around 41 percent of Carl Zeiss Meditec AG's shares are in free float. The remaining approx. 59 percent are held by Carl Zeiss AG,one of the world's leading groups in the optical and optoelectronic industries.
For more information visit our website at: www.zeiss.com/med

ZEISS CIRRUS 6000 including application

ZEISS EQ Workplace with screen without IOLMaster
Logo - http://cusmail.com/res/2023/07-22/19/658366966a2fabc5b0c5972ec84964b9.jpg
Photo - http://cusmail.com/res/2023/07-22/19/18dabbf73fdc289072db5ebdf7281465.jpg Photo - https://mma.prnewswire.com/media/1006943/ZEISS_CIRRUS_6000_incl_application.jpg
Photo - http://cusmail.com/res/2023/07-22/19/dfae3c59e8d8b564362178236b8a785b.jpg