Foresee-X Augment Reality Solution from SURGLASSES Is Registered with the FDA and Ready for Trauma Treatment
TAICHUNG,Taiwan,Dec. 30,2019 -- Foresee-X is a set of smart surgical glasses with functionality based on augmented and mixed reality technologies. This device was developed by SURGLASSES,also known as Taiwan Main Orthopaedic Biotechnology Co. Ltd.,and has received IEC60601-1-2 and ISO 13485 certifications.It is designed to bring a higher level of support to surgical procedures for trauma cases. SURGLASSES has provided Foresee-X to many surgeons and hospitals for clinical validation,and the product has been warmly received by the medical society. Recognizing that there is a strong potential demand for Foresee-X in the orthopaedic trauma market,SURGLASSES plans to begin mass production in 2020,sometime between the end of the first quarter and the start of the second quarter.
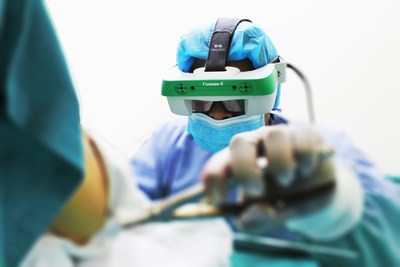
Foresee-X augment reality solution from SURGLASSES is registered with the FDA and ready for trauma treatment. (foto/SURGLASSES)
Targetingthe orthopaedic trauma market,Foresee-X helps surgeons by enhancing the synching of images from intra-operative fluoroscopy. This is especially beneficial for pelvic procedures,interlocking nail procedures,etc. Foresee-X is not only registered with the USFDA but also received the ISO 13485 certification (for quality management systems in medical devices) at the end of 2018. This set of smart surgical glasses features a novel augmented reality technology. Traditionally,surgeons performing trauma surgeries had to focus on both their patients and the various external monitors or screens in front of them at the same time. Foresee-X,which is equipped with an augmented reality solution for visual aid,also has image enhancement functions such as the ability to zoom in and out. The smart surgical glasses can thus increase efficiency by allowing surgeons to concentrate on the operational field instead of monitors. Foresee-X also reduces radiation exposure for surgeons by lowering the necessity of taking X-ray images during operation. It is thus a device that contributes to the safety of the medical staff and patients. Another advantage is that it improves accuracy by tracking the movements of surgical tools such as the puncture needle,trocar,etc. Additionally,Foresee-X allows outside observers to view procedures up close through tablet computers. The device can collect data for academic purposes as well.
Foresee-X is already being widely used by surgeons in Taiwan and other countries for trauma cases as part of a pre-market survey for obtaining valuable feedback from users. After receiving positive and informative product reviews from surgeons worldwide,SURGLASSES has further improved the functionality of the surgical smart glasses and plans to take the device to mass production. The upcoming version will fully meet the needs of surgeons and doctors working in the related fields. Foresee-X represents a breakthrough in orthopaedic surgery and a boon for patients. The device can help surgeons make smaller incisions than before,thus shortening the recovery time for patients.
SURGLASSES has been collaborating with hospitals in Taiwan and Malaysia to set up a specialized trauma center that includes Foresee-X as part of the equipment lineup. At this center,the smart surgical glasses are used for numerous kinds of orthopaedic procedures including interlocking of nails,pelvic cases,wrists,shoulders,tibia,and many more. With accuracy and efficiency as its main advantages,Foresee-X is the first of its kind on the market to provide cutting-edge assistance to surgeons and doctors dealing with trauma cases.

View original content to download multimedia:/news-releases/foresee-x-augment-reality-solution-from-surglasses-is-registered-with-the-fda-and-ready-for-trauma-treatment-300979685.html